55215-57-1
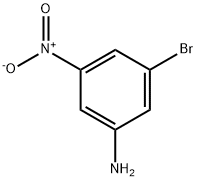 55215-57-1 结构式
55215-57-1 结构式
基本信息
3-溴-5-硝基苯胺
5-Bromo-3-nitroaniline
3-Bromo-5-nitroaniline96%
3-BroMo-5-nitrobenzenaMine
3-Bromo-5-nitroaniline 96%
3-Amino-5-bromonitrobenzene
Benzenamine, 3-bromo-5-nitro-
物理化学性质
制备方法
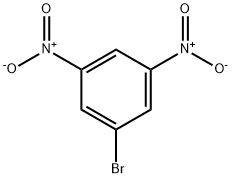
18242-39-2

55215-57-1
以3,5-二硝基溴苯为原料合成3-溴-5-硝基苯胺的一般步骤:在室温下,向1-溴-3,5-二硝基苯(3.0 g,12 mmol,1当量)的乙醇(15 mL)溶液中加入20%(NH4)2S水溶液(9.0 mL,26 mmol,2.2当量)。将反应混合物加热回流2小时。反应完成后,冷却至室温,用乙酸乙酯和水稀释。分离有机层,用饱和食盐水洗涤,减压浓缩。粗产物通过硅胶柱色谱纯化,采用乙酸乙酯/己烷(1:4)作为洗脱剂,得到2.2 g 3-溴-5-硝基苯胺(产率84%),为橙色固体。1H NMR (CDCl3, 300 MHz): δ 7.70 (s, 1H), 7.41 (d, J = 1.8 Hz, 1H), 7.08 (d, J = 1.5 Hz, 1H), 4.07 (br.s, 2H); m/z = 217 (M)+。
参考文献:
[1] Patent: US2011/130415, 2011, A1. Location in patent: Page/Page column 75-76
[2] Patent: WO2013/53983, 2013, A1. Location in patent: Page/Page column 49; 50
[3] Patent: US2015/11548, 2015, A1. Location in patent: Paragraph 0191
[4] Patent: CN103497211, 2016, B. Location in patent: Paragraph 0076; 0077
[5] Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), 1974, p. 1860 - 1862
