5731-01-1
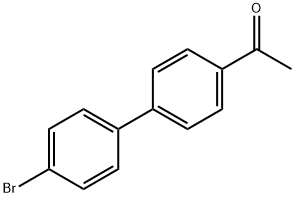 5731-01-1 结构式
5731-01-1 结构式
基本信息
4-乙酰基-4'-溴联苯
4-(2-溴苯基)苯乙酮
4-(4-BROMOPHENYL)ACETOPHENONE
4-ACETYL-4'-BROMOBIPHENYL
4-ACETYL-4'-BROMODIPHENYL
4'-(P-BROMOPHENYL)ACETOPHENONE
AKOS BAR-2458
TIMTEC-BB SBB005889
Acetylbromodiphenyl
1-(4'-bromo[1,1'-biphenyl]-4-yl)ethan-1-one
4-Acetyl-4'-bromobiphenyl 90%
4-(4-BROMOPHENYL)ACETOPHENONE 98%
4'-(p-Bromophenyl)acetophenone
1-(4'-Bromo-1,1'-biphenyl-4-yl)ethanone
4-Acetyl-4'-bromo-1,1'-biphenyl
4-Bromo-4'-acetylbiphenyl
物理化学性质
安全数据
Eye Irrit. 2
制备方法
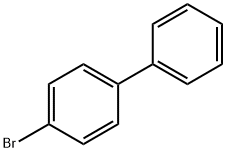
92-66-0

75-36-5

5731-01-1
一般步骤:在干燥的反应瓶中,将无水二氯甲烷(500 mL)加入无水氯化铝(26.66 g,199.7 mmol)中。将混合物搅拌并在盐/冰浴中冷却至5℃。通过滴液漏斗缓慢滴加乙酰氯(26.83 g,343.97 mmol),保持反应温度在-3℃,得到透明的黄色溶液。分批加入固体4-溴联苯(40 g,171.67 mmol),每次加入后搅拌30分钟,直至加完。将反应混合物在15℃下搅拌10小时。反应完成后,将混合物缓慢倒入冰水混合物中,并用37%盐酸淬灭反应。将混合物转移至分液漏斗中,用二氯甲烷萃取。有机相依次用200 mL水洗涤两次以去除盐酸,再用10%氢氧化钠溶液(200 mL)和水(200 mL)洗涤。合并有机层,用无水硫酸镁干燥,过滤除去干燥剂。减压蒸馏除去溶剂,残余物通过乙醇重结晶纯化。得到产物4-乙酰基-4'-溴代联苯(40.11 g,产率84.98%)。熔点:133.3℃。1H NMR (400 MHz, CDCl3) δ: 2.64 (s, 3H, CH3), 7.49 (d, J = 8.43 Hz, 2H), 7.59 (d, J = 8.80 Hz, 2H), 7.64 (d, J = 8.61 Hz, 2H), 8.03 (d, J = 8.25 Hz, 2H)。13C NMR (100.5 MHz, CDCl3) δ: 26.67 (CH3), 127.03, 128.81, 129.01, 132.09, 136.12, 138.76, 144.49, 197.62 (CO)。
参考文献:
[1] Journal of Molecular Liquids, 2012, vol. 175, p. 72 - 84
[2] Macromolecules, 2004, vol. 37, # 7, p. 2442 - 2449
[3] Molecular Crystals and Liquid Crystals Science and Technology, Section A: Molecular Crystals and Liquid Crystals, 1992, vol. 213, p. 187 - 206
[4] Journal of the Chemical Society, 1934, p. 869,871
[5] Journal of the Chemical Society [Section] C: Organic, 1966, p. 840 - 845
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2026/03/03 | L13798 | 4'-(4-溴苯基)苯乙酮, 97% 4'-(4-Bromophenyl)acetophenone, 97% | 5731-01-1 | 25g | 621元 |
| 2025/12/22 | XW02573101104 | 1-(4''-溴-[1,1''-联苯] 4-基)乙-1-酮 1-(4'-Bromo-[1,1'-biphenyl]-4-yl)ethan-1-one | 5731-01-1 | 100g | 1048元 |
| 2025/12/22 | XW02573101102 | 1-(4''-溴-[1,1''-联苯] 4-基)乙-1-酮 1-(4'-Bromo-[1,1'-biphenyl]-4-yl)ethan-1-one | 5731-01-1 | 10g | 118元 |

