5754-34-7
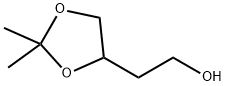 5754-34-7 结构式
5754-34-7 结构式
基本信息
4-(2-羟乙基)2,2-甲基-1,3-二氧戊环
4-(2-羟基)-2,2-二甲基-1,3-二氧戊环
4-(2-羟乙基)-2,2-二甲基-1,3-二氧戊环
4-(2-羟基乙基)-2,2-二甲基-1,3-二氧戊环
2,2-dimethyl-1,3-dioxolane-4-ethanol
1,3-Dioxolane-4-ethanol, 2,2-diMethyl-
4-(2-HYDROXYETHYL)-2,2-DIMETHYL-1,3-DIO&
4-(2-Hydroxyethyl)-2,2-diMethyl-1,3-dioxolaMe
4-(2-HYDROXYETHYL)-2,2-DIMETHYL-1,3-DIOXOLANE
(RAC)-2-(2,2-DIMETHYL[1,3]DIOXOLANE-4YL)ETHANOL
4-(2-Hydroxyethyl)-2,2-diMethyl-1,3-dioxolane 98%
物理化学性质
制备方法
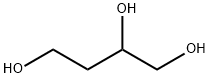
3068-00-6

67-64-1

5754-34-7
一般步骤:将1,2,4-丁三醇(15 g,141 mmol)、丙酮(100 mL,1362 mmol)和对甲苯磺酸一水合物(700 mg,3.68 mmol)的混合物在室温下搅拌16小时。反应完成后,向反应混合物中加入三乙胺(2 mL,14.3 mmol)以中和酸性催化剂,随后将混合物浓缩。所得残余物通过硅胶柱色谱法进行纯化(硅胶,洗脱溶剂:庚烷/乙酸乙酯= 1/0→1/1-1/3梯度),得到4-(2-羟基乙基)-2,2-二甲基-1,3-二氧戊环(16.55 g,收率:80.3%)作为无色油状物质。1H-NMR(400 MHz,CDCl3)δ ppm:1.37(3H,s),1.43(3H,s),1.79-1.86(2H,m),2.18-2.24(1H,br),3.60(1H,dd,J = 7.8 Hz),3.76-3.86(2H,m),4.10(1H,dd,J = 6.8 Hz),4.23-4.32(1H,m)。
参考文献:
[1] Nucleosides, Nucleotides and Nucleic Acids, 2005, vol. 24, # 10-12, p. 1543 - 1568
[2] European Journal of Organic Chemistry, 2015, vol. 2015, # 27, p. 6075 - 6083
[3] Journal of Organic Chemistry, 1996, vol. 61, # 3, p. 831 - 837
[4] Tetrahedron Letters, 1999, vol. 40, # 2, p. 209 - 212
[5] Bioorganic and Medicinal Chemistry Letters, 1999, vol. 9, # 9, p. 1247 - 1250
