651030-55-6
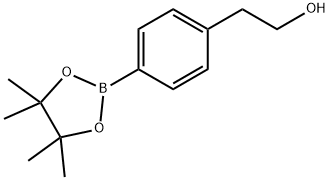 651030-55-6 结构式
651030-55-6 结构式
基本信息
4-(羟乙基)苯基硼酸频哪醇酯
4-(2-羟基乙基)苯硼酸频哪醇酯
2-[4-(四甲基-1,3,2-二氧杂硼烷-2-基)苯基]乙醇
2-(4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)苯基)乙醇
4-Hydroxyethylphenylboronic acid pinacol ester
4-(2-Hydroxyethyl)phenylboronic Acid Pinacol Ester
2-[4-(TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PHENYL]ETHANOL
2-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethan-1-ol
4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzeneethanol
Benzeneethanol, 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethanol
Benzeneethanol, 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-|||2-[4-(TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PHENYL]ETHANOL
物理化学性质
制备方法
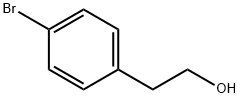
4654-39-1

73183-34-3

651030-55-6
步骤1:在氮气保护下,将4-溴苯乙醇(5.0g,25mmol)、联硼酸频那醇酯(7.6g,30mmol)、乙酸钾(4.88g,49.7mmol)和PdCl2(dppf)(0.91g,1.2mmol)溶于1,4-二恶烷(100mL)中。用氮气吹扫反应混合物后,在85℃下搅拌约12小时。反应完成后,冷却至室温,加入水(100mL)稀释,并用EtOAc(2×100mL)萃取。合并有机层,用饱和NaCl水溶液(100mL)洗涤,无水Na2SO4干燥,过滤后减压浓缩。残余物通过硅胶快速色谱法(17% EtOAc/石油醚)纯化。收集目标组分,减压浓缩,得到2-(4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)苯基)乙醇(6.2g,收率100%)。 NMR(400MHz, CDCl3)δ7.78(d, J=7.9Hz, 2H),7.29-7.23(m, 2H),3.87(t, J=6.6Hz, 2H),2.90(t, J=6.6Hz, 2H),1.36(s, 12H)。
参考文献:
[1] Patent: WO2016/168638, 2016, A1. Location in patent: Page/Page column 30; 31
[2] Patent: WO2016/168633, 2016, A1. Location in patent: Page/Page column 57
[3] Patent: WO2016/168641, 2016, A1. Location in patent: Page/Page column 105
[4] Journal of the American Chemical Society, 2014, vol. 136, # 42, p. 14742 - 14745
[5] Patent: EP1544194, 2005, A1. Location in patent: Page/Page column 37-38