69304-47-8
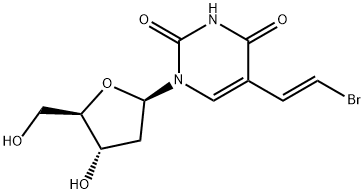 69304-47-8 结构式
69304-47-8 结构式
基本信息
溴夫啶
溴夫定
布里夫定
溴比夫定
布瑞夫定
溴乙烯尿苷
溴夫定/溴夫啶
溴夫定(标准品)
(E)-5-(2-溴乙烯基)-2'-脱氧核苷
BVDR
BrVDU
Helpin
Brivudin
SC-38394
BRIVUDINE
Brivudine BVdU
Brivudine USP/EP/BP
Bromovinyldeoxyuridine
物理化学性质
制备方法
![(2E)-3-[1-(2-脱氧-BETA-D-赤式戊呋喃糖基)-1,2,3,4-四氢-2,4-二氧代-5-嘧啶基]-2-丙烯酸](/CAS/GIF/74131-06-9.gif)
74131-06-9

69304-47-8
一般步骤:向(E)-5-(2-羧基乙烯基)-1-(2-脱氧-β-D-赤式戊呋喃糖基)嘧啶-2,4(1H,3H)-二酮(5.777 g,19.37 mmol)的二甲基甲酰胺(29 mL)溶液中加入K2CO3(5.890 g,42.61 mmol),并将该悬浮液在室温下搅拌15分钟。在20℃下,于30分钟内滴加N-溴代琥珀酰亚胺(3.655 g,20.53 mmol)的溶液。反应完成后,过滤所得悬浮液,并用DMF洗涤固体。将合并的滤液和洗涤液在真空下蒸发至干,残余物溶于甲醇中。向此溶液中加入硅胶,将悬浮液蒸发至干,然后将固体加载到色谱柱顶部。使用氯仿/甲醇(92:8,v/v)作为洗脱剂进行柱层析,得到白色固体(5.787 g,收率71.9%)。通过从水中重结晶得到白色粉末。产物的结构通过1H-NMR和13C-NMR确认:1H-NMR (DMSO-d6; 300 MHz) δ 11.59 (1H, bs, NH-3), 8.08 (1H, s, H-6), 7.25 (1H, d, 3J = 13.6 Hz, H-5B), 6.85 (1H, d, J = 13.6 Hz, H-5A), 6.13 (1H, t, 3J = 6.5 Hz, H-1'), 5.29 (1H, bs, OH-3'), 5.13 (1H, bs, OH-5'), 4.24 (1H, m, H-3'), 3.79 (1H, m, H-4'), 3.66 (2H, m, H-5'), 2.51 (1H, m, H-2'), 2.14 (1H, m, H-2'); 13C-NMR (DMSO-d6; 75 MHz) δ 40.2 (C-2'), 61.3 (C-5'), 70.3 (C-4'), 84.8 (C-3'), 87.8 (C-1'), 108.9 (C-5B), 110.0 (C-5), 130.3 (C-5A), 149.6, 162.1 (C-2, C-4)。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2005, vol. 13, # 24, p. 6663 - 6671
[2] RSC Advances, 2015, vol. 5, # 31, p. 24558 - 24563
[3] Patent: WO2005/12327, 2005, A2. Location in patent: Page/Page column 24-25
[4] Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 20, p. 5640 - 5642
[5] Patent: US6653318, 2003, B1
常见问题列表
溴夫定是一种抗病毒药物,于20世纪70年代由英国伯明翰大学Stanley Jones、Richard T. Walker团队合成。1978年,Erik De Clercq在第十二届欧洲生物化学学会联合会(FEBS)首次报道了溴夫定压倒性的抗单纯疱疹病毒(HSV)能力。1980年,Erik De Clercq发表了溴夫定首个用于治疗VZV感染临床研究。
溴夫定是一种核苷类似物,可以在病毒感染的细胞中经过磷酸化后形成5'-三磷酸溴夫定(BVDU TP),从而作为病毒DNA聚合酶的抑制剂和底物抑制DNA复制(见下图)。因溴夫定磷酸化过程由病毒胸苷激酶催化,只能在病毒感染的细胞的中进行,故具有高度选择性。再者,BVDU TP不再离开感染细胞,故在感染细胞中含量较高,具有迅速抑制病毒的作用。溴夫定具有极强的抗VZV活性,IC50为0.003μmol/L。
Brivudine is an analog of thymidine, and is incorporated into the viral DNA. It blocks the action of DNA polymerases, thus inhibiting viral replication. It has a stronger antiviral effect against the varicella-zoster virus compared with reference compounds such as aciclovir or penciclovir. It has high, selective activity against varicella zoster virus (VZV), inhibiting VZV replication, possibly through competitive inhibition of viral DNA polymerase, or by acting as an alternative substrate to deoxythymidine triphosphate, causing viral DNA strand breakage.
At a dose of 125 mg once daily, brivudine has proved to be superior to aciclovir with respect to reducing the period of new blister production, and has shortened the duration of post-herpetic neuralgia.