719310-31-3
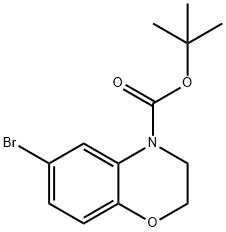 719310-31-3 结构式
719310-31-3 结构式
基本信息
6-溴-2H-苯并[B][1,4]噁嗪-4(3H)-羧酸叔丁酯
4-BOC-6-溴-3,4-二氢-2H-苯并[B][1,4]噁嗪
6-Bromo-4-Boc-2,3-dihydro-benzo[1,4]oxazine
tert-Butyl 6-bromo-2H-benzo[b][1,4]oxazine-4(3H)
4-Boc-6-bromo-3,4-dihydro-2H-benzo[b][1,4]oxazine
tert-butyl 6-bromo-2,3-dihydro-1,4-benzoxazine-4-carboxylate
tert-Butyl 6-broMo-2H-benzo[b][1,4]oxazine-4(3H)-carboxylate
tert-butyl 6-broMo-2,3-dihydrobenzo[b][1,4]oxazine-4-carboxylat
tert-butyl 6-broMo-2,3-dihydrobenzo[b][1,4]oxazine-4-carboxylate
6-bromo-2,3-dihydrobenzo[1,4]oxazine-4-carboxylic acid tert-butyl ester
4H-1,4-Benzoxazine-4-carboxylic acid, 6-broMo-2,3-dihydro-, 1,1-diMethylethyl ester
物理化学性质
制备方法

24424-99-5
![6-溴-3,4-二氢-2H-苯并[1,4]恶嗪盐酸盐](/CAS/GIF/105655-01-4.gif)
105655-01-4
![N-BOC-6-溴苯[B]并吗啉](/CAS/20180713/GIF/719310-31-3.gif)
719310-31-3
以6-溴-3,4-二氢-2H-苯并[b][1,4]恶嗪(1.37 g,6.4 mmol)和二碳酸二叔丁酯(1.676 g,7.68 mmol)为原料,在三乙胺(970 mg)和4-二甲氨基吡啶(78 mg,0.64 mmol)的存在下,于四氢呋喃(27 mL)中室温搅拌反应混合物过夜。反应完成后,将混合物用水(200 mL)稀释,并用乙酸乙酯(100 mL)萃取。有机层依次用水(50 mL)和盐水洗涤,无水硫酸钠干燥后浓缩,得到目标产物6-溴-2H-苯并[b][1,4]噁嗪-4(3H)-羧酸叔丁酯(1.3 g,产率65%)为黄色油状物。LC-MS分析显示[M-55]+峰位于m/z 258。1H NMR(400 MHz,DMSO-d6)δ 1.49(s,9H),3.78(t,J = 4.8 Hz,2H),4.21(t,J = 4.4 Hz,2H),6.83(d,J = 4.4 Hz,1H),7.12(dd,J1 = 2.0 Hz,J2 = 8.4 Hz,2H),8.01(s,1H)。
参考文献:
[1] Patent: WO2011/130628, 2011, A1. Location in patent: Page/Page column 227
[2] Patent: US2013/102595, 2013, A1. Location in patent: Paragraph 0575
[3] Patent: JP2015/187145, 2015, A. Location in patent: Paragraph 0488
[4] Patent: WO2008/44022, 2008, A1. Location in patent: Page/Page column 32
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0271931031303 | 6-溴-2H-苯并[b][1,4]噁嗪-4(3H)-羧酸叔丁酯 tert-Butyl 6-bromo-2,3-dihydrobenzo[b][1,4]oxazine-4-carboxylate | 719310-31-3 | 5g | 643元 |
| 2025/12/22 | XW0271931031302 | 6-溴-2H-苯并[b][1,4]噁嗪-4(3H)-羧酸叔丁酯 tert-Butyl 6-bromo-2,3-dihydrobenzo[b][1,4]oxazine-4-carboxylate | 719310-31-3 | 1g | 169元 |
| 2025/12/22 | XW0271931031301 | 6-溴-2H-苯并[b][1,4]噁嗪-4(3H)-羧酸叔丁酯 tert-Butyl 6-bromo-2,3-dihydrobenzo[b][1,4]oxazine-4-carboxylate | 719310-31-3 | 250mg | 54元 |