72040-64-3
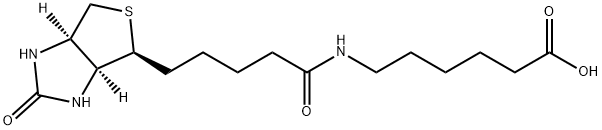 72040-64-3 结构式
72040-64-3 结构式
基本信息
生物素氨基己酸
6-生物素氨基己酸
生物素酰基-6-氨基己酸
生物素酰基-6-氨基己酸 1G
N-(+)-生物素基-6-氨基己酸
N-(+)-生物素酰基-6-氨基己酸
BIOTIN-LC
BIOTIN CAPROIC ACID
N-BIOTINYLCAPROIC ACID
D-BIOTIN-AMIDOCAPROIC ACID
6-BiotinaMidohexanoic Acid
Biotin-6-aMinohexanoic acid
(+)-Biotin-ω-aminocaproic acid
6-[(Biotinyl)amino]hexanoic acid
6-[(+)-BIOTINYL]AMINOCAPROIC ACID
物理化学性质
制备方法
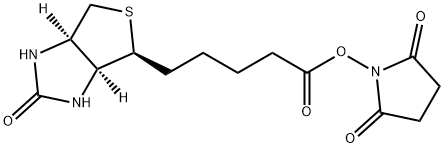
35013-72-0
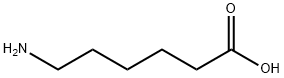
60-32-2

72040-64-3
以2,5-二氧代吡咯烷-1-基 5-((3aS,4S,6aR)-2-氧代六氢-1H-噻吩并[3,4-d]咪唑-4-基)戊酸酯和6-氨基己酸为原料,合成N-生物素己酸的一般步骤如下:将ε-氨基己酸(966 mg,8.4 mmol)溶解于30 mL 1M NaHCO3水溶液中。随后,向该溶液中缓慢滴加溶解于35 mL DMF中的N-羟基琥珀酰亚胺生物素(3.09 g),并在室温下搅拌反应混合物16小时。反应完成后,通过减压浓缩去除部分溶剂,然后加入150 mL柠檬酸水溶液(100 g/L),并在60℃下搅拌30分钟。过滤收集沉淀物,并用蒸馏水洗涤。将所得沉淀物溶解于异丙醇和水的混合溶剂(8:2,v/v)中,并于4℃下保存,最终得到纯的N-生物素己酸(1.455 g,产率50%),为淡黄色晶体。产物经IR(KBr)检测显示特征吸收峰:3200-3500, 3070, 2932, 2859, 1696, 1644, 1543, 1475, 1391, 1324, 1265, 1118 cm-1。1H NMR(600 MHz, DMSO-d6)数据:δ 7.75(1H, t, J = 5.4 Hz, C6-NH),6.45(1H, s, C13-NH),6.38(1H, s, C14-NH),4.35-4.27(1H, m, H-14),4.12(1H, m, H-13),3.09(1H, m, H-12),3.00(1H, m, H-6),2.82(1H, dd, J = 12.4, 5.1 Hz, H-15a),2.57(1H, d, J = 12.4 Hz, H-15b),2.19(2H, t, J = 7.4 Hz, H-2),2.04(2H, t, J = 7.3 Hz, H-8),1.20-1.67(14H, m)。13C NMR(150 MHz, DMSO-d6)数据:δ 171.84(C-1, C-7),162.74(C-16),61.06(C-13),59.20(C-14),55.44(C-12),39.68(C-15),38.25(C-6),35.21(C-8),29.05(C-2),28.92, 28.21, 28.04, 26.04, 25.73, 25.36。
参考文献:
[1] Chemical Communications, 2009, # 33, p. 5030 - 5032
[2] European Journal of Medicinal Chemistry, 2014, vol. 71, p. 219 - 228
[3] RSC Advances, 2018, vol. 8, # 57, p. 32775 - 32793
常见问题列表
Synthesis of
p
-nitrophenyl N-acetyllactosaminide with β-D-galactosidase, chemical reduction of the p-nitrophenyl group, and sialylation with sialyltransferase. The
p
-aminophenyl glycosides are then successfully biotin-labeled through the coupling with N-(+)-biotinyl-6- aminohexanoic acid to afford biotinylated oligosaccharides with an aminohexanosyl group and phenyl group as the spacers between the biotin and glycan.
The SERS (surface-enhanced Raman scattering) of cyclohexyl isocyanide that silver nanoparticles modified with N-(+)-biotinyl-6-aminocaproic acid and cyclohexyl isocyanide can be anchored on a separate biotinylated substrate via the avidin-biotin interaction.
