72551-53-2
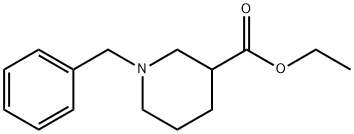 72551-53-2 结构式
72551-53-2 结构式
基本信息
ETHYL 1-BENZYLPIPERIDINE-3-CARBOXYLATE
1-Benzyl-piperidine-3-carboxylic acid ethal ester
1-Benzylpiperidine-3-carbonsSreethylester
Ethyl 1-benzylpiperidine-3-carboxylate 98%
物理化学性质
制备方法

100-39-0
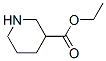
71962-74-8

72551-53-2
向哌啶-3-甲酸乙酯(5.94 mL,38.24 mmol,1.0当量)的二氯甲烷(80 mL)和水(40 mL)混合溶液中,依次加入溴化苄(4.77 mL,1.05当量)和碳酸钠(5.06 g,1.25当量)。将反应混合物加热回流3小时。反应完成后,分离有机层,用饱和食盐水洗涤,无水硫酸镁干燥,减压浓缩。残余物通过硅胶快速柱色谱纯化,以乙酸乙酯/己烷(1:6,v/v)为洗脱剂,得到1-苄基哌啶-3-甲酸乙酯(9),为无色油状物,收率87%。 1H NMR (400 MHz, CDCl3) δ 7.29-7.18 (m, 5H, Ar-H), 4.11 (q, J = 7.2 Hz, 2H, OCH2CH3), 3.51 (d, J = 13.6 Hz, 1H, CHa-Ph), 3.44 (d, J = 13.2 Hz, 1H, CHb-Ph), 2.90 (m, 1H, 哌啶-H), 2.68 (m, 1H, 哌啶-H), 2.54 (m, 1H, 哌啶-H), 2.20 (m, 1H, 哌啶-H), 2.01 (m, 1H, 哌啶-H), 1.90 (m, 1H, 哌啶-H), 1.69 (m, 1H, 哌啶-H), 1.52 (m, 2H, 哌啶-H), 1.19 (t, J = 7.2 Hz, 3H, OCH2CH3); 13C NMR (62.5 MHz, CDCl3): δ 174.4, 138.4, 129.1, 128.2, 127.0, 63.3, 60.3, 55.5, 53.7, 42.0, 27.0, 24.6, 14.2; HRESI-MS m/z calcd for [M + H]+ C15H22NO2: 248.1645, found: 248.1646.
参考文献:
[1] Journal of Medicinal Chemistry, 2003, vol. 46, # 25, p. 5512 - 5532
[2] Journal of Organic Chemistry, 2016, vol. 81, # 22, p. 11235 - 11249
[3] Tetrahedron Letters, 2001, vol. 42, # 33, p. 5645 - 5649
[4] European Journal of Medicinal Chemistry, 2017, vol. 127, p. 972 - 985
[5] Patent: US6664271, 2003, B1. Location in patent: Page column 15

