7298-67-1
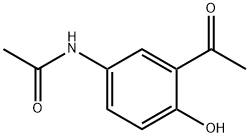 7298-67-1 结构式
7298-67-1 结构式
基本信息
2-羟基-5-乙酰胺基苯乙酮
2-羟基-5-乙酰氨基苯乙酮
5'-乙酰氨基-2'-羟基苯乙酮
2-羟基-5-乙酰胺基苯乙酮(成品)
N-(3-乙酰基-4-羟基苯基)乙酰胺
N1-(3-乙酰基-4-羟基苯基)乙酰胺
3'-Acetyl-4'-hydroxyacetanilide
5'-Acetamido-2'-hydroxypropiophenone
5'-AcetylaMino-2'-hydroxyacetophenone
N-(3-acetyl-4-hydroxyphenyl)acetamide
N1-(3-ACETYL-4-HYDROXYPHENYL)ACETAMIDE
5'-Acetamido-2'-hydroxyacetophenone >
Acetamide, N-(3-acetyl-4-hydroxyphenyl)-
物理化学性质
制备方法
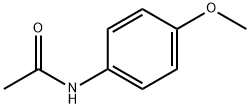
51-66-1

75-36-5

7298-67-1
以4-甲氧基乙酰苯胺和乙酰氯为原料合成2-羟基-5-乙酰氨基苯乙酮的一般步骤:将乙酰氯(25 mL,0.352 mol)缓慢滴加到4-甲氧基乙酰苯胺(20 g,0.121 mol)在二硫化碳(50 mL)中的搅拌悬浮液中。随后分批加入无水氯化铝(55 g,0.412 mol)。将反应混合物在80-90°C下加热搅拌90分钟。反应完成后,通过减压蒸馏除去溶剂。将残余物小心倒入碎冰和水的混合物中。过滤收集析出的固体,并用水充分洗涤。粗产物溶解于5%(w/v)的氢氧化钠水溶液中,溶液经Celite过滤。滤液用浓盐酸酸化至pH 1,析出2-羟基-5-乙酰氨基苯乙酮。过滤收集产物,用水洗涤,干燥后得白色固体(21.05 g,收率90%):熔点162-164°C;IR(KBr)ν:3452 cm-1(O-H伸缩振动),1657 cm-1(C=O伸缩振动);1H NMR(DMSO-d6, 500 MHz):δ 2.02(s,3H,-NHCOCH3),2.58(s,3H,-COCH3),6.90(d,1H,5-H),7.65(dd,1H,6-H),8.06(s,1H,2-H),9.89(s,1H,-NHCOCH3),11.54(s,1H,-OH)。元素分析(C10H11NO3)计算值:C,62.17;H,5.74;N,7.25。实测值:C,62.35;H,5.82;N,7.19。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 13, p. 3919 - 3928
[2] Patent: WO2012/107262, 2012, A1. Location in patent: Page/Page column 29
[3] Journal of Organic Chemistry, 1995, vol. 60, # 14, p. 4324 - 4330
[4] Journal of Medicinal Chemistry, 1992, vol. 35, # 21, p. 3973 - 3976
[5] Bioorganic and Medicinal Chemistry, 2004, vol. 12, # 9, p. 2079 - 2098
