73087-83-9
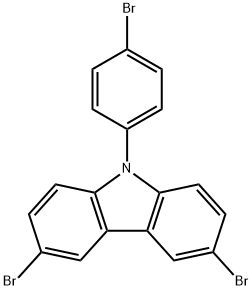 73087-83-9 结构式
73087-83-9 结构式
基本信息
3,6-二溴-9-(4-溴苯基)咔唑
9-(4-溴苯基)-3,6-二溴-9H-咔唑
3,6-二溴-9-(4-溴苯基)-9H-咔唑
3,6-Dibromo-9-(4-bromophenyl)carbazole>
9-(4-Bromophenyl)-3,6-dibromo-9H-carbazole
3,6-Dibromo-9-(4-bromo-phenyl)-9H-carbazole
9H-Carbazole, 3,6-dibromo-9-(4-bromophenyl)-
9H-Carbazole, 3,6-dibroMo-9-(4-broMophenyl)- 3,6-DibroMo-9-(4-broMophenyl)carbazole
物理化学性质
制备方法
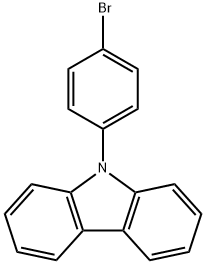
57102-42-8

73087-83-9
以9-(4-溴苯基)咔唑为原料合成3,6-二溴-9-(4-溴苯基)-9H-咔唑的一般步骤: 1. 将9-(4-溴苯基)-9H-咔唑(0.1g,0.31mmol,1当量)溶解于DMF中,制备成溶液。 2. 在搅拌下,缓慢滴加N-溴代琥珀酰亚胺(0.12g,0.69mmol,2.2当量)的DMF溶液(3.5mL)至上述溶液中。 3. 将反应混合物在室温下持续搅拌3小时。 4. 反应完成后,向混合物中加入水,析出白色沉淀。 5. 通过己烷重结晶纯化沉淀,得到白色固体产物(0.082g,收率55%)。 产物表征数据: 1H NMR (CDCl3, 400 MHz) δ 7.20 (d, 2H, J = 8.5 Hz), 7.37 (d, 2H, J = 8.6 Hz), 7.50 (dd, 2H, J = 1.9, 8.7 Hz), 7.73 (d, 2H, J = 8.5 Hz)。 13C NMR (100 MHz, CDCl3) δ 139.8, 136.0, 133.5, 129.7, 128.7, 124.2, 123.5, 121.9, 113.5, 111.4。 HRMS (TOF MS ES+): 计算值C18H10Br3N [M]+ 478.8338,实测值478.8349。
参考文献:
[1] Organic and Biomolecular Chemistry, 2016, vol. 14, # 10, p. 2961 - 2968
[2] Patent: CN103360416, 2016, B. Location in patent: Paragraph 0039; 0040
[3] Journal of Materials Chemistry, 2010, vol. 20, # 37, p. 8126 - 8133
[4] Synlett, 2006, # 17, p. 2841 - 2845
[5] Dyes and Pigments, 2013, vol. 96, # 3, p. 705 - 713
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | D4563 | 3,6-二溴-9-(4-溴苯基)咔唑 3,6-Dibromo-9-(4-bromophenyl)carbazole | 73087-83-9 | 1g | 1880元 |
| 2025/05/22 | D4563 | 3,6-二溴-9-(4-溴苯基)咔唑 3,6-Dibromo-9-(4-bromophenyl)carbazole | 73087-83-9 | 200mg | 400元 |
| 2025/05/22 | XW730878392 | 3,6-Dibromo-9-(4-bromophenyl)-9H-carbazole 3,6-Dibromo-9-(4-bromophenyl)-9H-carbazole | 73087-83-9 | 1g | 33元 |
