73842-99-6
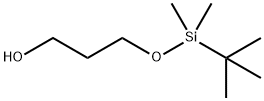 73842-99-6 结构式
73842-99-6 结构式
基本信息
3-(叔丁基二甲基硅氧基)丙醇
3-[(叔丁基二甲基硅烷基)氧基]丙醇
3-((叔丁基二甲基甲硅烷基)氧)丙醇
3-(叔丁基二甲基硅氧基)丙醇 25G
3-((输丁基二甲基甲硅烷基)氧)-丙醇
3-(T-BUTYLDIMETHYLSILOXY)PROPANOL
3-(T-Butyldimethylsiloxy)propan-1-ol
3-(TERT-BUTYLDIMETHYLSILOXY)PROPANOL
3-[(TERT-BUTYLDIMETHYLSILYL)OXY]PROPANOL
3-[(TERT-BUTYLDIMETHYLSILYL)OXY]-1-PROPANOL
3-(tert-ButyldiMethylsilanyloxy)propan-1-ol
3-((tert-ButyldiMethylsilyl)oxy)propan-1-ol
3-((tert-ButyldiMethylsilyl)oxy)-propanol 97%
3-[Dimethyl(1,1-dimethylethyl)siloxy]propanol
物理化学性质
制备方法

18162-48-6

504-63-2

73842-99-6
以叔丁基二甲基氯硅烷和1,3-丙二醇为原料合成3-(叔丁基二甲基硅氧基)丙醇的一般步骤如下:该合成作为制备dictyostatin及其类似物的示例性“底部片段”15的一部分,已在文献中报道。具体而言,1,3-丙二醇经过基于Evans手性辅助的方法,经过九个步骤转化为已知的双-TBS保护的Homer-Wadsworth-Emmons产物10。相关文献参见:Phukan, P.; Sasmal, S.; Maier, M. E. Eur. J. Org. Chem. 2003, 1733以及Andrus, M. B.; Argade, A. B. Tetrahedron Lett. 1996, 37, 5049。
参考文献:
[1] Patent: WO2005/117588, 2005, A2. Location in patent: Page/Page column 31
[2] Journal of the American Chemical Society, 2004, vol. 126, # 37, p. 11440 - 11441
[3] Organic Letters, 2007, vol. 9, # 16, p. 3105 - 3108
[4] Patent: WO2014/145302, 2014, A2. Location in patent: Page/Page column 34; 35; 38; 39
[5] Patent: WO2016/44558, 2016, A1. Location in patent: Page/Page column 51; 52; 54; 55
