747414-17-1
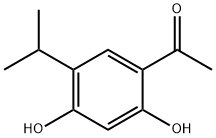 747414-17-1 结构式
747414-17-1 结构式
基本信息
4-乙酰基-6-异丙基-1,3-苯二酚
1-(2,4-二羟基-5-异丙基苯基)乙酮
乙酮, 1-[2,4-二羟基-5-(异丙基)苯基]-
2’,4’-Dihydroxy-5’-isopropylacetophenone
1-(2,4-Dihydroxy-5-isopropylphenyl)ethanone
1-(2,4-dihydroxy-5-propan-2-ylphenyl)ethanone
1-(2,4-dihydroxy-5-isopropylphenyl)ethan-1-one
Ethanone, 1-[2,4-dihydroxy-5-(1-Methylethyl)phenyl]-
1-(2,4-Dihydroxy-5-isopropylphenyl)ethanone USP/EP/BP
物理化学性质
制备方法
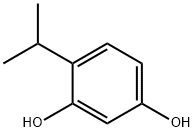
23504-03-2

64-19-7

747414-17-1
首先,在配备有两个250 mL烧瓶和回流冷凝器的装置中,加入4-异丙基苯-1,3-二醇(I-4,5.36 g,35.25 mmol)。在氮气保护下,缓慢注入100 mL的47%三氟化硼乙醚溶液,持续半小时。随后,加入4.03 mL的冰醋酸,并在油浴中加热回流过夜。反应完成后,通过薄层色谱(TLC)监测反应进程。待反应混合物冷却至室温后,加入70 mL的10%乙酸钠水溶液,搅拌2小时。将混合物用水稀释至250 mL,然后用乙酸乙酯萃取三次。合并有机层,通过旋转蒸发去除溶剂。有机相用无水硫酸钠干燥,过滤后得到红棕色固体。将该固体在二氯甲烷中浆化,加入后产生浅黄色不溶物。通过玻璃漏斗抽滤,并用二氯甲烷洗涤,最终得到淡黄色固体6.80 g,即目标化合物I-5,产率为99.4%。
参考文献:
[1] Patent: CN105439972, 2016, A. Location in patent: Paragraph 0094; 0095; 0107; 0108; 0109
[2] Journal of Medicinal Chemistry, 2008, vol. 51, # 2, p. 196 - 218
[3] Patent: CN103724269, 2016, B. Location in patent: Paragraph 0388; 0389; 0403; 0404; 0405; 0406
[4] Patent: US2009/76006, 2009, A1. Location in patent: Page/Page column 32; 41
[5] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 16, p. 3129 - 3134
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0274741417103 | 4-乙酰基-6-异丙基-1,3-苯二酚 1-(2,4-Dihydroxy-5-isopropylphenyl)ethanone | 747414-17-1 | 1g | 330元 |
| 2025/12/22 | XW0274741417102 | 4-乙酰基-6-异丙基-1,3-苯二酚 1-(2,4-Dihydroxy-5-isopropylphenyl)ethanone | 747414-17-1 | 250mg | 138元 |
| 2025/12/22 | XW0274741417101 | 4-乙酰基-6-异丙基-1,3-苯二酚 1-(2,4-Dihydroxy-5-isopropylphenyl)ethanone | 747414-17-1 | 100mg | 56元 |
