78818-15-2
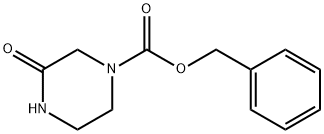 78818-15-2 结构式
78818-15-2 结构式
基本信息
3-OXOPIPERAZINE-1-CARBOXYLIC ACID BENZYL ESTER
4-BENZYLOXYCARBONYL-2-PIPERAZINONE
4-BENZYLOXYCARBONYLPIPERAZIN-2-ONE
4-CBZ-PIPERAZINONE
4-Z-PIPERAZIN-2-ONE
BENZYL 3-OXOPIPERAZINE-1-CARBOXYLATE
BENZYL 3-OXOTETRAHYDRO-1(2H)-PYRAZINECARBOXYLATE
BUTTPARK 32\04-69
N'-Z-PIPERAZIN-2-ONE
Piperazin-2-one, N4-CBZ protected
1-CBZ-3-OXOPIPERAZINE
N-CBZ-PIPERAZINONE
N-CARBOXYBENZYLOXYPIPERAZINONE
3-Oxopiperazine-1-carboxylic acid benzyl ester, 4-Benzyloxycarbonyl-2-piperazinone
物理化学性质
安全数据
制备方法
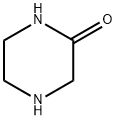
5625-67-2

501-53-1

78818-15-2
步骤2: 3-氧代哌嗪-1-羧酸苄酯的合成 在0℃下,将碳酸钠(24.77g,233.73mmol,3.00当量)和哌嗪-2-酮(7.80g,77.91mmol,1.00当量)溶于乙基乙酸酯(70.00mL)和水(70.00mL)的混合溶剂中。随后,缓慢加入氯甲酸苄酯(16.79g,93.49mmol,13.99mL,纯度95%,1.20当量)。将反应混合物在30℃下搅拌16小时,通过TLC监测反应进度。反应完成后,用乙酸乙酯(80mL×3)萃取混合物。合并有机层,用饱和氯化钠水溶液(80mL×3)洗涤,无水硫酸钠干燥,减压浓缩得到粗产物。粗产物通过打浆纯化(石油醚:乙酸乙酯= 20:1,80mL),在30℃下搅拌15分钟后过滤。固体经真空干燥,得到4-苄氧羰基-2-哌嗪酮(15.50g,66.17mmol,收率84.93%),为白色固体。
参考文献:
[1] Journal of Organic Chemistry, 1991, vol. 56, # 11, p. 3715 - 3719
[2] Tetrahedron, 2010, vol. 66, # 18, p. 3370 - 3377
[3] Patent: EP3269715, 2018, A1. Location in patent: Paragraph 0238; 0239
[4] European Journal of Medicinal Chemistry, 2017, vol. 132, p. 26 - 41
[5] European Journal of Medicinal Chemistry, 1981, vol. 16, # 3, p. 229 - 232
