79261-58-8
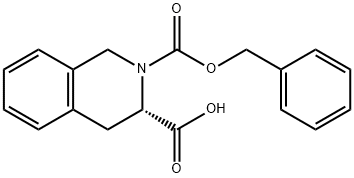 79261-58-8 结构式
79261-58-8 结构式
基本信息
(3S)-2-CARBOBENZOXY-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID
(3S)-2-CBZ-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID
N-ALPHA-CARBOBENZOXY-L-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID
(S)-(+)-2-(BENZYLOXYCARBONYL)-1,2,3,4-TETRAHYDRO-3-ISOQUINOLINECARBOXYLIC ACID
(S)-(+)-N-CBZ-1,2,3,4-TERTRAHYDRO ISOQUINOLINE-3-CARBOXYLIC ACID
Z-[3S]-1,2,3,4-TETRAHYDROISOQUINOLENE-3-CARBOXYLIC ACID
Z-D-[3S]-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID
Z-L-1,2,3,4-TETRAHYDROISOQUINOLINE-3-CARBOXYLIC ACID
Z-TIC-OH
(S)-(+)-2-(BENZYLOXYCARBONYL)-TETRAHY-DR O-3-ISOQUINOLINECARBOXYLIC ACID, 97%
(S)-N-Benzyloxycarbonyl-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid
物理化学性质
制备方法
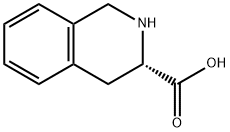
74163-81-8

501-53-1

79261-58-8
以(S)-(-)-1,2,3,4-四氢异喹啉-3-羧酸和氯甲酸苄酯为原料合成(S)-(+)-N-苄氧羰基-1,2,3,4-四氢异喹啉-3-羧酸的一般步骤:向1M的NaOH水溶液(3.20g,80.0mmol,溶于80mL H2O中)中,在室温下缓慢加入(S)-1,2,3,4-四氢异喹啉-3-羧酸(5.00g,28.2mmol)和二恶烷,直至悬浮液完全溶解。随后,在0℃下,于30分钟内缓慢滴加氯甲酸苄酯(6.25g,5.59mL,36.7mmol)。反应混合物在室温下搅拌16小时。通过蒸发去除二恶烷,反应混合物用1M HCl在0℃下酸化至pH 2。水层用EtOAc萃取,合并的有机层经Na2SO4干燥,过滤后真空蒸发,得到目标产物(S)-(+)-N-苄氧羰基-1,2,3,4-四氢异喹啉-3-羧酸(8.68g,27.9mmol,收率99%),为白色泡沫状固体,无需进一步纯化。产物经1H-NMR、13C-NMR、MS和LC-MS表征,确认其结构和纯度。
参考文献:
[1] Journal of Medicinal Chemistry, 2016, vol. 59, # 5, p. 2222 - 2243
[2] Patent: WO2017/63910, 2017, A1. Location in patent: Page/Page column 20-21
[3] Journal of Organic Chemistry, 2001, vol. 66, # 23, p. 7575 - 7587
[4] Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 17, p. 4371 - 4373
[5] Journal of Organic Chemistry, 2016, vol. 81, # 3, p. 956 - 968
知名试剂公司产品信息
(3S)-2-[Benzyloxycarbonyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, 97%(79261-58-8)
