80655-81-8
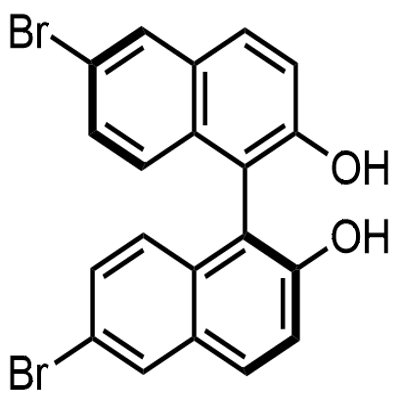 80655-81-8 结构式
80655-81-8 结构式
基本信息
(S)-(+)-6,6"-二溴-1,1"-2-联萘酚
6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(+/-)-6,6'-DIBROMO-2,2'-DIHYDROXY-1,1'-BINAPHTHYL
6'-DIBROMO-1,1'-BI-2-NAPHTHOL
AURORA KA-7214
(R)-(-)-6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(R)-(+)-6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(R)-(-)-6,6'-DIBROMO-1,1'-BI-NAPHTHOL
(R)-(-)-6,6'-DIBROMO 1,1'-BL-2-NAPHTHOL
(R)-(-)-6,6'-DIBROMO-2,2'-DIHYDROXY-1,1'-BINAPHTHYL
(R)-(-)-6'-DIBROMO-1,1'-BI-2-NAPHTHOL
RACEMIC-6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(S)-(+)-6,6'-DIBROM-2,2'-DIHYDROXY-1,1'-BINAPHTHYL
(S)-(-)-6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(S)-(+)-6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(S)-6,6'-DIBROMO-1,1'-BI-2-NAPHTHOL
(S)-(+)-6,6'-DIBROMO-1,1'-BI-NAPHTHOL
(S)-6,6'-DIBROMO-1,1'-BINAPHTHYL-2,2'-DIOL
(S)-(+)-6,6'-DIBROMO-2,2'-DIHYDROXY-1,1'-BINAPHTHYL
(S)-(+)-6'-DIBROMO-1,1'-BI-2-NAPHTHOL
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
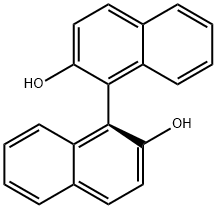
18531-99-2
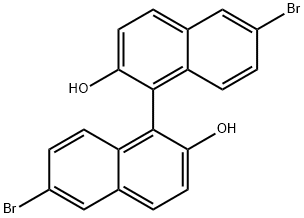
13185-00-7
以(S)-1,1'-联-2-萘酚为原料合成(S)-6,6'-二溴-1,1'-联-2-萘酚的一般步骤:在-78℃下,将(S)-BINOL(7.20 g,25.0 mmol,1.0当量)悬浮于二氯甲烷(250 mL)中。将溴(3.9 mL,34.0 mmol,1.4当量)在二氯甲烷(40 mL)中的溶液缓慢滴加到反应混合物中(滴加时间20-30分钟),并在-78℃下继续搅拌15分钟。使反应混合物缓慢升温至室温,并通过薄层色谱(TLC)监测反应进程(约3小时),直至反应完全。加入饱和硫代硫酸钠水溶液(50 mL)淬灭反应,水层用乙酸乙酯(3×50 mL)萃取。合并有机层,用无水硫酸镁干燥,过滤后减压浓缩除去溶剂。产物通过二氯甲烷/戊烷重结晶,得到(S)-6,6'-二溴-1,1'-联-2-萘酚,为白色固体(10.34 g,23.3 mmol,收率93%)。1H NMR (300 MHz, CDCl3) δ: 8.03 (d, J = 1.9 Hz, 2H), 7.87 (d, J = 9.0 Hz, 2H), 7.45-7.35 (m, 4H), 6.94 (d, J = 9.0 Hz, 2H), 5.00 (bs, 2H). 13C NMR (75 MHz, CDCl3) δ: 153.0, 131.9, 130.8, 130.7, 130.6, 130.4, 125.9, 119.0, 118.0, 110.7。分析数据与文献报道一致。
参考文献:
[1] Journal of the American Chemical Society,
[2] Journal of the American Chemical Society, 2009, vol. 131, p. 3621 - 3630
[3] Angewandte Chemie - International Edition, 2002, vol. 41, # 7, p. 1159 - 1162
[4] Chemical Communications, 2010, vol. 46, # 27, p. 4911 - 4913
[5] Chemical Communications, 2012, vol. 48, # 54, p. 6851 - 6853
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | D2730 | (S)-(+)-6,6'-二溴-1,1'-联-2-萘酚 (S)-(+)-6,6'-Dibromo-1,1'-bi-2-naphthol | 80655-81-8 | 1G | 790元 |
| 2025/12/22 | XW028065581802 | (S)-(+)-6,6''-二溴-1,1''-联-2-萘酚 (S)-(-)-6,6'-Dibromo-1,1'-bi-2-naphthol | 80655-81-8 | 1g | 102元 |
| 2025/12/22 | D2730 | (S)-(+)-6,6'-二溴-1,1'-联-2-萘酚 (S)-(+)-6,6'-Dibromo-1,1'-bi-2-naphthol | 80655-81-8 | 5g | 2895元 |
