80793-25-5
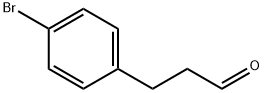 80793-25-5 结构式
80793-25-5 结构式
基本信息
3-(4-溴苯基)丙醛
4-(4-broMophenyl)butanal
Benzenepropanal, 4-broMo-
3-(4-BROMO-PHENYL)-PROPIOLDEHYDE
3-(4-BROMO-PHENYL)-PROPIONALDEHYDE
物理化学性质
制备方法
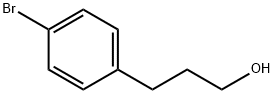
25574-11-2

80793-25-5
一般步骤:实施例A.4:3-(4-溴苯基)丙醛(10×)的合成 将4-溴苯丙醇(4.71 g,21.8 mmol)溶解于二氯甲烷(DCM)中,加入Dess-Martin periodinane(9.71 g,20.9 mmol),室温下反应2小时。反应完成后,用2M NaOH溶液淬灭反应,分离有机相和水相,水相用DCM萃取。合并有机相,用无水硫酸镁(MgSO4)干燥,过滤后减压浓缩。粗产物通过硅胶快速色谱法纯化,洗脱剂为环己烷(cHex)中DCM的梯度溶液,得到目标产物3-(4-溴苯基)丙醛(10×)(4.46 g,收率96%),为无色液体。 1H NMR(400 MHz,CDCl3)δ 9.81(s, 1H),7.41(d, J = 8.4 Hz, 2H),7.07(d, J = 8.4 Hz, 2H),2.91(t, J = 7.6 Hz, 2H),2.79-2.74(m, 2H)。 MS(ES)C9H9BrO计算值:211/213,([M-H]-)未检测到,纯度100%。
参考文献:
[1] Patent: WO2015/181272, 2015, A1. Location in patent: Page/Page column 30
[2] Patent: EP3239143, 2017, A2. Location in patent: Paragraph 0120
[3] Patent: WO2015/66413, 2015, A1. Location in patent: Page/Page column 176; 177
[4] Patent: US2017/253554, 2017, A1. Location in patent: Paragraph 0425; 0426
[5] Chemical Communications, 2011, vol. 47, # 27, p. 7875 - 7877
