82578-45-8
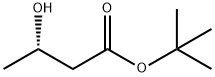 82578-45-8 结构式
82578-45-8 结构式
基本信息
叔-丁基 (S)-(+)-3-羟基丁酸酯
叔-丁基(S)-(+)-3-羟基丁酸酯,98%
Tert-butyl (S)-3-hydroxybutanoate
(S)-tert-Butyl 3-hydroxybutanoate
tert-butyl (3S)-3-hydroxybutanoate
(S)-(+)-T-BUTYL 3-HYDROXYBUTANOATE
(+)-TERT-BUTYL (S)-3-HYDROXYBUTYRATE
(S)-(+)-TERT-BUTYL 3-HYDROXYBUTYRATE
tert-Butyl(S)-(+)-3-hydroxybutyrate,98%
(S)-3-HYDROXY-BUTYRIC ACID TERT-BUTYL ESTER
Butanoic acid,3-hydroxy-,1,1-dimethylethyl ester,(S)-
物理化学性质
制备方法
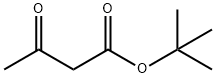
1694-31-1

82578-45-8
以乙酰乙酸叔丁酯为原料合成(S)-3-羟基丁酸叔丁酯的一般步骤如下:酮1-7的实验室放大生物还原按以下步骤进行。发酵72小时后,将R. arrhizus菌丝体与培养液分离。将10%湿菌丝体悬浮于1.5L灭菌的新鲜培养基中,置于5L Erlenmeyer烧瓶内,在无菌条件下作为工作体积,于室温静态孵育72小时。真菌生长后,将底物(1g)的乙醇溶液直接加入培养基中,随后在室温下于旋转振荡器(100rpm)上培养8天。孵育结束后,通过过滤分离菌丝体。菌丝体用水洗涤,合并的水相用氯仿萃取。氯仿萃取液用水洗涤后,用Na2SO4干燥。减压除去溶剂后,按前述方法分离、纯化并表征产物醇。绝对构型通过比旋光符号确定,并与文献数据对比。分离产率:0.81g,[α]D26 = +28.6(c 0.483,CHCl3),>99%ee {文献值:[α]D26 = +32.3(c 1.03,CHCl3),99.0%ee};1H NMR(CDCl3, 200MHz):δ1.25(d, 3H, CH3),1.48(s, 9H, CH3),2.27-2.49(m, 2H, CH2),3.09(s, 1H, OH),4.08-4.22(m, 1H, CHOH);13C NMR(CDCl3, 200MHz, ppm):22.21, 27.93, 43.79, 64.18, 80.94, 172.15;MTPA酯的甲氧基共振,1H NMR:δ3.55[主要,(S)-异构体]。
参考文献:
[1] RSC Advances, 2016, vol. 6, # 34, p. 28447 - 28450
[2] Tetrahedron Letters, 2006, vol. 47, # 27, p. 4619 - 4622
[3] Tetrahedron Asymmetry, 2008, vol. 19, # 19, p. 2272 - 2275
[4] Tetrahedron: Asymmetry, 2016, vol. 27, # 4-5, p. 188 - 192
[5] Tetrahedron Asymmetry, 1997, vol. 8, # 7, p. 1049 - 1054
