848488-91-5
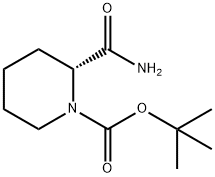 848488-91-5 结构式
848488-91-5 结构式
基本信息
N-BOC-D-2-哌啶甲酰胺
N-叔丁氧羰基-D-2-哌啶甲酰胺
(R)-2-氨基甲酰基哌啶-1-羧酸叔丁酯
N-Boc-D-2-piperidinecarboxamide
(R)-1-Boc-2-piperidinecarboxaMide
(R)-1-Boc-2-(aminocarbonyl)piperidine
tert-Butyl (R)-2-carbamoylpiperidine-1-carboxylate
(R)-tert-butyl 2-carbamoylpiperidine-1-carboxylate
(R)-tert-Butyl 2-carbamoyllpiperidine-1-carboxylate
(R)-2-Carbamoyl-piperidine-1-carboxylic acid tert-butyl ester
1-Piperidinecarboxylic acid, 2-(aminocarbonyl)-, 1,1-dimethylethyl ester, (2R)-
制备方法
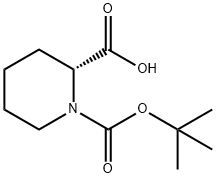
28697-17-8

848488-91-5
步骤B. (2R)-2-(氨基羰基)哌啶-1-羧酸叔丁酯的合成: 在0℃下,向(2R)-1-(叔丁氧基羰基)哌啶-2-甲酸(2.29 g, 10 mmol)、氯化铵(3.21 g, 60 mmol)和DMF(70 mL)的混合物中加入HATU(5.60 g, 14.7 mmol)和DIPEA(3.88 g, 30 mmol)。将反应混合物在室温下搅拌18小时。反应完成后,用H2O(100 mL)稀释反应液,并用EtOAc(3×100 mL)萃取。合并有机相,依次用10% Na2CO3溶液(2×30 mL)、盐水(2×30 mL)洗涤,然后用Na2SO4干燥。过滤并浓缩后,通过MPLC在硅胶上使用己烷/EtOAc(1:1)作为洗脱剂进行纯化,得到R-N-Boc-脯氨酰胺,为白色固体。1H NMR (400 MHz, CDCl3) δ: 1.46 (s, 9H), 1.63 (m, 2H), 2.22 (m, 1H), 2.91 (m, 1H), 3.06 (m, 3H), 4.01 (m, 1H), 4.71 (m, 1H), 6.46 (br, 2H)。MS (ESI) m/z: 228.92 (M+H)+。
参考文献:
[1] Patent: WO2005/115986, 2005, A1. Location in patent: Page/Page column 103
[2] Patent: WO2006/116764, 2006, A1. Location in patent: Page/Page column 121; 151
[3] Patent: US2008/300279, 2008, A1. Location in patent: Page/Page column 9
[4] ACS Infectious Diseases, 2018, vol. 4, # 7, p. 1130 - 1145

