849758-12-9
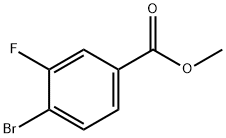 849758-12-9 结构式
849758-12-9 结构式
基本信息
4-bromo-3-fluorobenzoate methyl
4-bromo-3-fluoro-2-methylbenzoate
METHYL 4-BROMO-3-FLUOROBENZOATE 98
Methyl 4-bromo-3-fluorobenzoate 98%
Benzoicacid, 4-broMo-3-fluoro-, Methyl ester
物理化学性质
制备方法

67-56-1
153556-42-4

849758-12-9
步骤2:在氮气保护下,将1-甲基哌嗪(980μL,8.80mmol)溶于甲苯(15mL)中,依次加入三(二亚苄基丙酮)二钯(201mg,0.22mmol)、BINAP(550mg,0.88mmol)和碳酸铯(4.30g,13.2mmol)。将反应混合物在室温下搅拌30分钟后,加入4-溴-3-氟苯甲酸甲酯(2.05g,8.80mmol)。随后,将反应体系加热至105℃,持续搅拌18小时。反应完成后,将混合物冷却至室温,通过硅藻土垫过滤,并用乙酸乙酯洗涤滤饼。合并滤液,减压浓缩,得到橙色油状粗产物。粗产物通过硅胶柱色谱法纯化,以5%甲醇的二氯甲烷溶液作为洗脱剂。收集目标馏分,减压浓缩,得到3-氟-4-(4-甲基哌嗪-1-基)苯甲酸甲酯(1.80g,81%)为无色油状物。产物经1H NMR(400MHz,CD3OD)和质谱(EI)确认结构:1H NMR (400MHz, CD3OD): δ 7.78-7.73 (d, 1H), 7.64-7.59 (d, 1H), 7.09-7.03 (m, 1H), 3.86 (s, 3H), 3.26-3.20 (m, 4H), 2.65-2.59 (m, 4H), 2.35 (s, 3H). MS (EI): m/z 253 (MH+).
参考文献:
[1] Patent: US2010/216806, 2010, A1. Location in patent: Page/Page column 103
[2] Patent: WO2009/55077, 2009, A1. Location in patent: Page/Page column 369
[3] Patent: US2009/23748, 2009, A1. Location in patent: Page/Page column 21
[4] Patent: US2012/230951, 2012, A1. Location in patent: Page/Page column 97-98
[5] Patent: WO2016/20836, 2016, A1. Location in patent: Page/Page column 101; 102
