85259-19-4
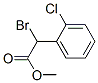 85259-19-4 结构式
85259-19-4 结构式
基本信息
A-溴代邻氯苯乙酸甲酯
alpha-溴-2-氯苯乙酸甲酯
2-溴-2-(2-氯苯基)乙酸甲酯
A-溴代邻氯苯乙酸甲酯,氯吡格雷中间体
A-溴代邻氯苯乙酸甲酯,氯吡格雷中间体 1KG
Methyl a-Bromo-2-chlorophenylacetate
Methyl a-Bromo-2-chlorobenzeneacetat
Methyl a-Bromo-2-chlorobenzeneacetate
Methyl α-Bromo-2-chlorobenzeneacetate
Methyl alpha-bromo-2-chlorophenylacetate
a-Bromo-2-chlorobenzeneacetic Acid Methyl Ester
α-Bromo-2-chloro benzeneacetic Acid Methyl Ester
2-Bromo-2-(2-chlorophenyl)acetic acid methyl ester
alpha-Bromo-2-chloro-phenylacetic acid methyl ester
制备方法
57486-68-7

85259-19-4
以邻氯苯基乙酸甲酯为原料合成α-溴-2-氯苯基乙酸甲酯的一般步骤:向搅拌的2-氯苯基乙酸甲酯(0.86 mL, 5.31 mmol)的二氯甲烷(10.2 mL, 159 mmol)溶液中依次加入N-溴代琥珀酰亚胺(1.04 g, 5.84 mmol)和偶氮二异丁腈(43 mg)。在氩气保护下,将反应混合物于100℃搅拌反应16小时。反应完成后,将混合物冷却至室温,用乙醚稀释并过滤。滤液经减压浓缩除去溶剂,得到的油状残余物含有琥珀酰亚胺(456 mg),用庚烷稀释后再次过滤。最终,通过减压蒸馏除去溶剂,得到α-溴-2-氯苯基乙酸甲酯(1.38 g, 4.56 mmol),产率为86%。产物经1H NMR(500 MHz, CDCl3)表征:δ 7.69(dd, J = 7.6, 1.8 Hz, 1H),7.31(dd, J = 7.6, 1.7 Hz, 1H),7.24(td, J = 7.6, 1.7 Hz, 1H),7.21(dd, J = 7.5, 1.8 Hz, 1H),5.84(s, 1H),3.74(s, 3H)。
参考文献:
[1] Patent: WO2018/149856, 2018, A1. Location in patent: Paragraph 93-94
[2] Patent: WO2018/149853, 2018, A1. Location in patent: Page/Page column 96
[3] Angewandte Chemie - International Edition, 2013, vol. 52, # 16, p. 4440 - 4444
[4] Angew. Chem., 2013, vol. 125, # 16, p. 4536 - 4540,5
[5] Journal of Medicinal Chemistry, 1993, vol. 36, # 23, p. 3738 - 3742
