870281-85-9
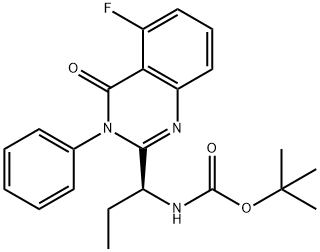 870281-85-9 结构式
870281-85-9 结构式
基本信息
IDELALISIB N-2中间体
CAL101 IDELALISIB N-2中间体
CAL-101(N-2)IDELALISIB N-2中间体
二氢-4-氧代-3-苯基-2-喹唑啉基)丙基]氨基甲酸叔丁酯
CAL-101(N-2)IDELALISIB N-2中间体 250G
(S)-(1-(5-氟-4-氧代-3-苯基-3,4-二氢喹唑啉-2-基)丙基)氨基甲酸叔丁酯
[(1S)-1-(5-氟-3,4-二氢-4-氧代-3-苯基-2-喹唑啉基)丙基]氨基甲酸叔丁酯
[(1S)-1-(5-氟-3, 4-二氢-4-氧代-3-苯基-2-喹唑啉YL) 丙基]-氨基甲酸1, 1-二甲基乙酯
tert-butyl (S)-[1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl]carbamate
(S)-tert-butyl (1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)carbaMate
(S)-tert-butyl (1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)carbaMate(CAL101 N-2 step)
[(1S)-1-(5-Fluoro-3,4-dihydro-4-oxo-3-phenyl-2-quinazolinyl)propyl]carbamic acid 1,1-dimethylethyl ester
(S)-tert-butyl (1-(5-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)carbaMate, [(1S)-1-(5-Fluoro-3,4-dihydro-4-oxo-3-phenyl-2-quinazolinyl)propyl]carbamic acid 1,1-dimethylethyl ester
制备方法
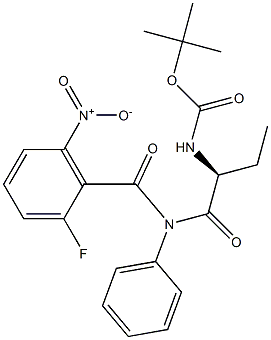
870281-84-8

870281-85-9
3. 制备(S)-[1-(5-氟-4-氧代-3-苯基-3,4-二氢喹唑啉-2-基)丙基]氨基甲酸叔丁酯 在200 mL反应烧瓶中,将化合物(V)(8.9 g,0.02 mol)溶于100 mL乙酸中,搅拌至完全溶解。向反应体系中分批加入锌粉(9.1 g,0.14 mol),控制反应温度为25°C,持续搅拌3小时。反应完成后,通过真空过滤收集反应混合物,并用乙酸(50 mL)洗涤滤饼。将滤液浓缩,滤饼用二氯甲烷(100 mL)和水(50 mL)洗涤。随后,用二氯甲烷(50 mL)萃取水层,合并有机相,依次用饱和碳酸钠溶液(50 mL)和饱和盐水(50 mL)洗涤。有机层用无水硫酸镁干燥,浓缩后得到白色固体化合物(VI),即(S)-[1-(5-氟-4-氧代-3-苯基-3,4-二氢喹唑啉-2-基)丙基]氨基甲酸叔丁酯(6.2 g),产率为78%,纯度为95%。
参考文献:
[1] Patent: CN104130261, 2016, B. Location in patent: Paragraph 0055; 0056; 0057; 0058
[2] Patent: WO2005/113554, 2005, A2. Location in patent: Page/Page column 24-25; 27-28
[3] Organic Preparations and Procedures International, 2016, vol. 48, # 4, p. 337 - 341
[4] Patent: WO2016/108206, 2016, A2. Location in patent: Page/Page column 47; 48
[5] RSC Advances, 2018, vol. 8, # 28, p. 15863 - 15869
