876343-09-8
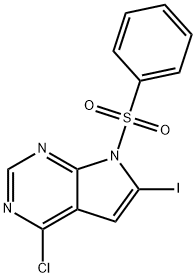 876343-09-8 结构式
876343-09-8 结构式
基本信息
7-(苯磺酰基)-4-氯-6-碘-吡咯并[2,3-D]嘧啶
4-氯-6-碘-7-(苯基磺酰基)-7H吡咯并[2,3]嘧啶
7-(苯磺酰)-4-氯-6-碘-7H-吡咯并[2,3-D]嘧啶
7-(苯磺酰基)-4-氯-6-碘-7H-吡咯并[2,3-D]嘧啶
4-氯-6-碘-7-(苯基磺酰基)-7H-吡咯并[2,3-D]嘧啶
4-CHLORO-6-IODO-7-PHENYLSULFONYL-7H-PYRROLO[2,3-D]PYRIMIDINE 4-氯-6-碘-7-(苯基磺酰基)-7H-吡咯并[2,3-D]嘧啶
7-(Benzenesulfonyl)-4-chloro-6-iodo-pyrrolo[2,3-d]pyrimidine
4-Chloro-6-iodo-7-phenylsulfonyl-7H-pyrrolo[2,3-d]pyrimidine
7-(benzenesulfonyl)-4-chloro-6-iodo-7H-pyrrolo[2,3-d]pyrimidine
7H-Pyrrolo[2,3-d]pyrimidine, 4-chloro-6-iodo-7-(phenylsulfonyl)-
物理化学性质
制备方法
![4-氯-7-(苯基磺酰基)-7h-吡咯并[2,3-d]嘧啶](/CAS/GIF/186519-89-1.gif)
186519-89-1
![7-(苯磺酰基)-4-氯-6-碘-7H-吡咯并[2,3-D]嘧啶](/CAS2/GIF/876343-09-8.gif)
876343-09-8
以4-氯-7-(苯磺酰基)-7H-吡咯并[2,3-d]嘧啶为原料合成4-氯-6-碘-7-(苯基磺酰基)-7H-吡咯并[2,3]嘧啶的一般步骤:在氮气保护下,将4-氯-7-(苯磺酰基)-7H-吡咯并[2,3-d]嘧啶(10.0g,34.0mmol)溶于无水THF(300ml)中,冷却至-78℃。使用注射泵在30分钟内缓慢滴加二异丙基氨基锂(2M的THF/正庚烷/乙苯溶液,26mL,51.1mmol)。反应1小时后,再使用注射泵在30分钟内滴加溶解于无水THF(60ml)中的碘(11.2g,44.3mmol)。反应混合物继续搅拌1小时,随后加入1M HCl溶液(180ml)。搅拌至反应混合物温度升至22℃,然后进行浓缩。向浓缩物中加入二氯甲烷(250ml)和水(200ml),分离有机层。水相用二氯甲烷(2×90ml)萃取,合并有机相后用盐水(100ml)洗涤,无水硫酸钠干燥,过滤并浓缩。粗产物通过乙腈(150ml)重结晶,得到浅黄色固体产物4-氯-6-碘-7-(苯基磺酰基)-7H-吡咯并[2,3]嘧啶(10.5g,25.1mmol,收率74%),熔点为190℃(分解)。产物纯度经HPLC测定为99%(保留时间t R = 25.9分钟)。1H NMR(400MHz,DMSO-d6)δ:8.77(s,1H),8.12-8.09(m,2H),7.82-7.78(m,1H),7.71-7.67(m,2H),7.34(s,1H);13C NMR(100MHz,DMSO-d6)δ:153.3, 152.1, 150.0, 137.2, 135.5, 130.0(2C), 127.6(2C), 120.6, 116.6, 84.6;IR(neat,cm-1):1394, 1087, 724;HRMS(EI,m/z):418.8988(计算值:C12H7ClIN3S,418.8987,[M]+)。
参考文献:
[1] ChemMedChem, 2014, vol. 9, # 11, p. 2516 - 2527
[2] Patent: WO2015/959, 2015, A1. Location in patent: Page/Page column 62-63
[3] Patent: WO2018/88780, 2018, A1. Location in patent: Page/Page column 13
[4] Patent: WO2015/89327, 2015, A1. Location in patent: Paragraph 0264
[5] Patent: WO2006/17443, 2006, A2. Location in patent: Page/Page column 56
