882670-69-1
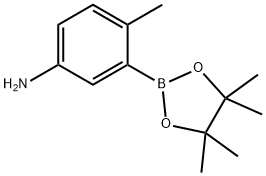 882670-69-1 结构式
882670-69-1 结构式
基本信息
5-氨基-2-甲基苯硼酸匹那醇酯
5-氨基-2-甲基苯硼酸频那醇酯
5-氨基-2-甲基苯硼酸频哪醇酯
5-氨基-2-甲基苯基硼酸频那醇酯
4-甲基-3-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)苯胺
4,4,5,5-四甲基-2-(5-氨基-2-甲基苯基)-1,3,2-二氧杂硼杂环戊烷
5-Amino-2-methylphenylboronic acid pinacol ester 97%
4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)anil...
4-Methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
4,4,5,5-TetraMethyl-2-(5-aMino-2-Methylphenyl)-1,3,2-dioxaborolane
4-Methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzenamine
4-Methyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine
Benzenamine, 4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
3-Hydroxy-2,3-dimethylbutan-2-yl hydrogen 5-amino-2-methylphenylboronate
5-Amino-2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-benzenamine
物理化学性质
安全数据
Skin Irrit. 2
制备方法
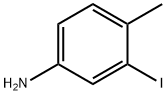
35944-64-0

73183-34-3

882670-69-1
以3-碘-4-甲基苯胺(1.97g,8.45mmol)和联硼酸频那醇酯(2.32g,9.13mmol)为原料,在氩气保护下,将联硼酸频那醇酯溶于DMSO(23mL)中,依次加入乙酸钾(2.87g,29.2mmol)和[1,1'-双(二苯基膦基)二茂铁]二氯钯(345mg,0.423mmol)。将反应混合物在80℃下搅拌4小时。反应完成后,向混合物中加入乙酸乙酯和水,通过硅藻土过滤除去不溶物,用乙酸乙酯萃取有机相。有机层依次用水和盐水洗涤,无水硫酸镁干燥。减压浓缩除去溶剂,残余物通过硅胶柱色谱(洗脱剂:乙酸乙酯/己烷=6/1)纯化,得到目标产物4-甲基-3-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)苯胺(化合物A4,1.36g,收率69%)。产物经1H NMR(300MHz,CDCl3)表征:δ 1.33(s,12H),2.42(s,3H),3.51(br s,2H),6.68(dd,J = 8.1,2.7Hz,1H),6.97(d,J = 8.1Hz,1H),7.11(d,J = 2.7Hz,1H)。
参考文献:
[1] Patent: EP2269993, 2011, A1. Location in patent: Page/Page column 52
[2] Journal of Medicinal Chemistry, 2006, vol. 49, # 19, p. 5671 - 5686
[3] Patent: US2010/197688, 2010, A1. Location in patent: Page/Page column 16
[4] Patent: WO2015/24905, 2015, A1. Location in patent: Paragraph 00216-00217; 00344-00346
[5] Patent: US2015/80391, 2015, A1. Location in patent: Paragraph 0439; 0440; 0441; 0655; 0656; 0657
