893441-85-5
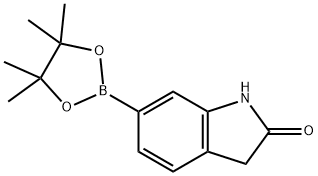 893441-85-5 结构式
893441-85-5 结构式
基本信息
2-氧代吲哚啉-6-硼酸频哪醇酯
6-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-2-吲哚酮
2-Oxoindoline-6-boronic Acid Pinacol Ester
6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)indolin-2-one
6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2H-indol-2-one
6-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-1,3-dihydro-indol-2-one
2H-Indol-2-one, 1,3-dihydro-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
物理化学性质
制备方法
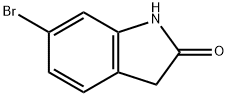
99365-40-9

73183-34-3

893441-85-5
以6-溴-2-吲哚酮和联硼酸频那醇酯为原料合成2-氧代吲哚啉-6-硼酸频哪醇酯的一般步骤如下:准备四个微波反应瓶,分别加入6-溴二氢吲哚-2-酮(500 mg,2.36 mmol)、双(频哪醇合)二硼(898 mg,3.54 mmol)、乙酸钾(694 mg,7.07 mmol)和Pd(dppf)Cl2·CH2Cl2(96.0 mg,0.118 mmol)。将上述混合物溶解于1,2-二甲氧基乙烷(DME,17 mL)中。将反应体系在80℃下加热反应过夜。反应完成后,合并四个反应瓶中的内容物,浓缩反应液,并通过柱色谱法(环己烷/乙酸乙酯作为洗脱剂)进行纯化,得到目标化合物2-氧代吲哚啉-6-硼酸频哪醇酯,为白色固体(2.27 g,收率75%,纯度80%)。产物结构经1H NMR(500 MHz, CDCl3)确认:δ 8.57(宽单峰,1H),7.48(双峰,J=7.3 Hz,1H),7.31(单峰,1H),7.23(双峰,J=7.3 Hz,1H),3.55(单峰,2H),1.33(单峰,12H)。LC-MS(ESI, m/z)分析显示:保留时间Rt=2.75 min,分子离子峰m/z=260([M+H]+,HPLC方法E)。
参考文献:
[1] Patent: WO2015/144290, 2015, A1. Location in patent: Page/Page column 85
[2] Patent: WO2015/39172, 2015, A1. Location in patent: Page/Page column 60
[3] Patent: WO2011/15641, 2011, A1. Location in patent: Page/Page column 128
[4] Patent: WO2011/101640, 2011, A1. Location in patent: Page/Page column 106
[5] Patent: WO2008/23161, 2008, A1. Location in patent: Page/Page column 120