893566-74-0
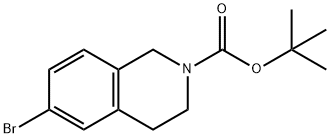 893566-74-0 结构式
893566-74-0 结构式
基本信息
N-BOC-6-溴-1,2,3,4-四氢异喹啉
2-BOC-6-溴-1,2,3,4-四氢异喹啉
6-溴-3,4-二氢异喹啉-2(1H)-甲酸叔丁酯
6-溴-3,4-二氢-2(1H)-异喹啉羧酸-1,1-二甲基乙酯
2-Boc-6-bromo-3,4-dihydro-1H-isoquinoline
2-Boc-6-bromo-1,2,3,4-tetrahydroisoquinoline
tert-Butyl 6-bromo-3,4-dihydroisoquinoline-2(1H)
tert-butyl 6-bromo-3,4-dihydro-1H-isoquinoline-2-carboxylate
6-bromo-2-(tert-butoxycarbonyl)-1,2,3,4-tetrahydroisoquinoline
tert-butyl 6-broMo-1,2,3,4-tetrahydroisoquinoline-2-carboxylate
6-BROMO-3,4-DIHYDRO-1H-ISOQUINOLINE-2-CARBOXYLIC ACID TERT-BUTYL ESTER
2(1H)-Isoquinolinecarboxylic acid, 6-bromo-3,4-dihydro-, 1,1-dimethylethyl ester
物理化学性质
制备方法
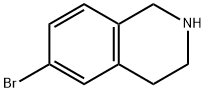
226942-29-6

24424-99-5

893566-74-0
在室温条件下,将二碳酸二叔丁酯(3.52 g,16.14 mmol)缓慢加入至6-溴-1,2,3,4-四氢异喹啉(3.26 g,15.37 mmol)的四氢呋喃(45 mL)溶液中。反应混合物在室温下持续搅拌15小时。反应完成后,通过减压蒸馏除去溶剂。粗产物通过硅胶柱色谱法进行纯化,采用溶剂梯度洗脱(0→8%乙酸乙酯/己烷),得到目标化合物6-溴-3,4-二氢异喹啉-2(1H)-甲酸叔丁酯(5.05 g,16.18 mmol,定量收率)为无色油状物。1H NMR(300 MHz,CDCl3)δ:1.49(9H,s),2.80(2H,t,J = 5.9 Hz),3.62(2H,t,J = 5.9 Hz),4.51(2H,s),6.97(1H,d,J = 8.7 Hz),7.28-7.32(2H,m)。
参考文献:
[1] Patent: EP3192791, 2017, A1. Location in patent: Paragraph 0594
[2] ChemMedChem, 2018, vol. 13, # 14, p. 1405 - 1413
[3] Patent: WO2013/80222, 2013, A1. Location in patent: Page/Page column 40
[4] Patent: WO2017/184662, 2017, A1. Location in patent: Page/Page column 58
[5] Patent: WO2017/68412, 2017, A1. Location in patent: Page/Page column 274
