89856-44-0
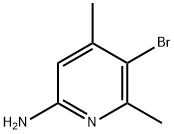 89856-44-0 结构式
89856-44-0 结构式
基本信息
5-溴-4,6-二甲基吡啶-2-胺
2-氨基-5-溴-4,6-二甲基吡啶
6-氨基-3-溴-2,4-二甲基吡啶
3-Bromo-2,4-dimethylpyridin-6-amine
5-bromo-4,6-dimethyl-2-pyridinamine
5-BroMo-4,6-diMethylpyridin-2-aMine
2-AMino-4,6-diMethyl-5-broMopyridine
6-Amino-2,4-dimethyl-3-bromopyridine
2-Pyridinamine, 5-bromo-4,6-dimethyl-
2-AMino-5-broMo-4,6-diMethylpyridine 97%
2-AMINO-5-BROMO-4 6-DIMETHYLPYRIDINE& ISO 9001:2015 REACH
5-Bromo-4,6-dimethyl-2-pyridinamine, 6-Amino-3-bromo-2,4-lutidine
物理化学性质
安全数据
Eye Dam. 1
Skin Sens. 1
制备方法
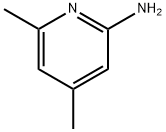
5407-87-4

89856-44-0
实施例2:2-氨基-5-溴-4,6-二甲基吡啶(13)的制备:在氩气保护下,将2.00g(16.32mmol)2-氨基-4,6-二甲基吡啶溶于25mL乙腈中,搅拌下加入2.90g(16.32mmol)N-溴代琥珀酰亚胺。反应混合物于室温下搅拌5小时。反应完成后,过滤收集生成的沉淀,干燥后得到目标产物2-氨基-5-溴-4,6-二甲基吡啶,为白色固体,产量2.76g(产率84%)。产物结构经1H-NMR(CDCl3)δ6.22(s,1H),4.39(br,2H),2.48(s,3H),2.25(s,3H)和13C-NMR(CDCl3)δ156.34,155.23,148.64,112.26,108.10,25.10,23.30确认。
参考文献:
[1] Organic Process Research and Development, 2012, vol. 16, # 1, p. 109 - 116
[2] Patent: WO2011/103536, 2011, A1. Location in patent: Page/Page column 43-44
[3] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 17, p. 5188 - 5201
[4] Pharmacy and Pharmacology Communications, 1999, vol. 5, # 3, p. 233 - 238
[5] Patent: WO2018/108671, 2018, A1. Location in patent: Page/Page column 178; 179
常见问题列表
2-氨基-5-溴-4,6-二甲基吡啶为固体化合物。

