939-69-5
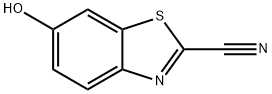 939-69-5 结构式
939-69-5 结构式
基本信息
6-羟基-2-氰基苯并噻唑
2-氰基-6-羟基苯并噻唑
6-羟基苯并[D]噻唑-2-腈
6-羟基苯并[D]噻唑-2-甲腈
2-cyano-6-hydroxybenzothiazoL
2-CYANO-6-HYDROXYBENZOTHIAZOLE
6-Hydroxy-Benzothiazole-2-Carb
2-Cyano-6-hydroxy-1,3-benzothiazole
2-Cyano-6-hydroxybenzothiazole, >=95%
6-Hydroxy-2-Benzothiazolecarbonitrile
2-Benzothiazolecarbonitrile, 6-hydroxy
6-hydroxy-benzothiazole-2-carbonitrile
6-Hydroxybenzothiazole-2-carbonitrile 96%
物理化学性质
制备方法
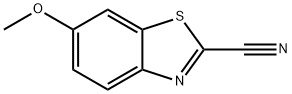
943-03-3

939-69-5
以2-氰基-6-甲氧基苯并噻唑为原料合成2-氰基-6-羟基苯并噻唑的一般步骤:在氩气保护下,将吡啶盐酸盐(2.32 g)加入至2-氰基-6-甲氧基苯并噻唑(51.4 mg,0.271 mmol)中,加热至200℃使吡啶盐酸盐完全溶解,持续搅拌反应混合物30分钟。反应完成后,冷却反应混合物,随后加入1M盐酸溶液(50 mL)。用乙酸乙酯(3×50 mL)进行萃取,合并有机层并用无水硫酸钠干燥。干燥后的有机层经减压浓缩,得到粗产物。通过制备型薄层硅胶色谱法纯化(使用20 cm×20 cm×1.75 mm的硅胶板,展开剂为己烷-乙酸乙酯(1:1)),得到2-氰基-6-羟基苯并噻唑(47.2 mg,收率99%)为浅黄色固体。1H NMR(270 MHz,CD3OD)δ 7.17(1H,dd,J = 2.7, 9.2 Hz),7.41(1H,d,J = 2.7 Hz),7.99(1H,d,J = 9.2 Hz)。
参考文献:
[1] Patent: EP2754657, 2014, A1. Location in patent: Paragraph 0088-0090
[2] Patent: US5616729, 1997, A
[3] Organic and Biomolecular Chemistry, 2015, vol. 13, # 7, p. 2117 - 2121
[4] Chemistry - A European Journal, 2016, vol. 22, # 27, p. 9330 - 9337
[5] Patent: JP6095208, 2017, B2. Location in patent: Paragraph 0048-0050
