947249-13-0
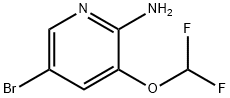 947249-13-0 结构式
947249-13-0 结构式
基本信息
5-溴-3-(二氟甲氧基)吡啶-2-胺
5-bromo-3-(difluoromethoxy)-2-Pyridinamine
5-Bromo-3-(difluoromethoxy)pyridin-2-amine
5-bromo-3-(difluoromethoxy)pyridine-2-amine
2-Pyridinamine, 5-bromo-3-(difluoromethoxy)-
物理化学性质
制备方法
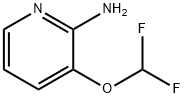
947249-14-1

947249-13-0
以3-(二氟甲氧基)吡啶-2-胺为原料合成2-氨基-3-(二氟甲氧基)-5-溴吡啶的一般步骤:在0℃下,将N-溴代琥珀酰亚胺(2.61g,14.65mmol)在3分钟内缓慢加入至3-(二氟甲氧基)吡啶-2-胺(2.3g,14.36mmol)的乙腈溶液(15mL)中。反应混合物在相同温度下继续搅拌20分钟。随后,将反应液真空浓缩至干,所得粘性物质用水稀释,并用乙酸乙酯(3×60mL)萃取。合并有机层,用无水硫酸钠干燥,再次浓缩至干。通过柱层析(硅胶,100-200目,20%乙酸乙酯的己烷溶液作为洗脱剂)纯化残余物,得到目标产物5-溴-3-(二氟甲氧基)吡啶-2-胺(3.2g,收率93%)。产物经核磁共振氢谱(400 MHz,DMSO-d6)确认:δ7.89(s,1H),7.51(s,1H),7.16(t,J = 73.6Hz,1H),6.34(s,2H)。
参考文献:
[1] Patent: WO2014/111496, 2014, A1. Location in patent: Page/Page column 108; 109
[2] Patent: WO2015/91889, 2015, A1. Location in patent: Page/Page column 74, 75
[3] Patent: US2015/175619, 2015, A1. Location in patent: Paragraph 0221; 0222
[4] Patent: WO2009/16460, 2009, A2. Location in patent: Page/Page column 56
[5] Patent: US2013/210818, 2013, A1. Location in patent: Paragraph 0416
