405224-22-8
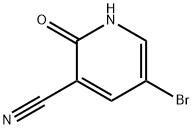 405224-22-8 结构式
405224-22-8 结构式
基本信息
5-溴-2-羟基吡啶-3-甲腈
5-溴-2-羟基-3-氰基吡啶
5-溴-3-氰基-2-羟基吡啶
3-氰基-5-溴-2(1H)-吡啶酮
5-溴-3-氰基-2(1H)-吡啶酮
5-溴-3-氰基-2-羟基吡啶,5-溴-2-羟基烟腈
5-溴-3-氰基-2(1H)-吡啶酮,5-溴-3-氰基-2-羟基吡啶
5-Bromo-3-cyano-2(1H)-pyridinone
5-BROMO-2-HYDROXYNICOTINONITRILE
5-BROMO-3-CAYNO-2(1H)-PYRIDINONE
5-BROMO-3-CYANO-2-HYDROXYPYRIDINE
5-Bromo-2-hydroxynicotinonitrile98%
5-Bromo-2-hydroxynicotinonitrile 98%
5-Bromo-3-cyano-2-hydroxypyridine 98%
5-bromo-2-hydroxypyridine-3-carbonitrile
5-BROMO-2-HYDROXY-3-PYRIDINECARBONITRILE
物理化学性质
制备方法
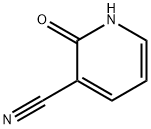
20577-27-9

405224-22-8
以2-氧代-1,2-二氢吡啶-3-腈为原料合成5-溴-2-氧代-1,2-二氢吡啶-3-甲腈的一般步骤:在室温下,将2-氧代-1,2-二氢吡啶-3-腈(50mg,0.42mmol)和乙酸钠(69mg,0.84mmol)溶解于5mL冰乙酸中,搅拌至完全溶解。随后,向该溶液中缓慢加入溴(21.5μL,0.42mmol),反应持续2小时。反应完成后,将混合物在真空下浓缩以除去乙酸。通过柱色谱法(以乙酸乙酯为洗脱剂)纯化粗产物,得到5-溴-2-氧代-1,2-二氢吡啶-3-甲腈(69mg,产率75%),为黄色固体。熔点:216~217℃。1H NMR(400MHz,CD3COCD3):δ11.70(s,1H),8.22(d,J = 2.8Hz,1H),8.05(d,J = 2.8Hz,1H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 16, p. 3251 - 3255
[2] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 23, p. 6578 - 6581
[3] Bioorganic and Medicinal Chemistry Letters, 2003, vol. 13, # 9, p. 1577 - 1580
[4] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 20, p. 5578 - 5585
[5] Patent: US2004/19052, 2004, A1. Location in patent: Page/Page column 17
