95470-42-1
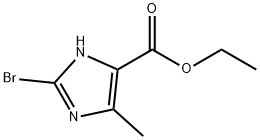 95470-42-1 结构式
95470-42-1 结构式
基本信息
2-溴-5-甲基-1H-咪唑-4-甲酸乙酯
2-溴-4-甲基-1H-咪唑-5-羧酸乙酯
2-溴-5-甲基-1H-咪唑-4-羧酸乙酯
Ethyl 2-bromo-4-methyl-1H-imidazole-5-carboxylate
Ethyl 2-broMo-5-Methyl-4H-iMidazole-4-carboxylate
ethyl 2-broMo-5-Methyl-1H-iMidazole-4-carboxylate
2-Bromo-5-methyl-4H-imidazole-4-carboxylic acid ethyl ester
2-BROMO-5-METHYL-1H-IMIDAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
2-BROMO-5-METHYL-3H-IMIDAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
1H-Imidazole-5-carboxylic acid,2-bromo-4-methyl-,ethyl ester
1H-Imidazole-4-carboxylic acid, 2-bromo-5-methyl-, ethyl ester
物理化学性质
制备方法
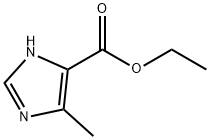
51605-32-4

95470-42-1
一般步骤:以4-甲基-5-咪唑甲酸乙酯为原料合成2-溴-4-甲基-1H-咪唑-5-羧酸乙酯(EV-AT8648-001)的操作如下: 步骤1:向搅拌的4-甲基-1H-咪唑-5-羧酸乙酯(CAS 51605-32-4,500 mg,3.24 mmol)在乙腈(10 mL)和氯仿(10 mL)的混合溶液中,加入N-溴代琥珀酰亚胺(577 mg,3.24 mmol)。反应在氮气保护下,于室温搅拌进行18小时。反应完成后,将反应混合物浓缩,残余物通过快速柱色谱法(洗脱剂:10-100%乙酸乙酯/庚烷梯度)纯化,得到560 mg(产率73%)目标产物2-溴-4-甲基-1H-咪唑-5-羧酸乙酯(EV-AT8648-001),为灰白色固体。 LCMS分析(方法D):保留时间0.87分钟,质谱m/z = 233/235([M+H]+)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 12, p. 3669 - 3674
[2] Patent: WO2017/147102, 2017, A1. Location in patent: Paragraph 00167-00168
[3] Patent: WO2008/111794, 2008, A1. Location in patent: Page/Page column 27
[4] Patent: US2009/156615, 2009, A1. Location in patent: Page/Page column 19
[5] Patent: US2015/284358, 2015, A1. Location in patent: Paragraph 0126; 0127; 0128
