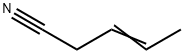
3-PENTENENITRILE
- CAS No.
- 4635-87-4
- Chemical Name:
- 3-PENTENENITRILE
- Synonyms
- 3-Pent;crotyl cyanide;3-PENTENONITRILE;3-PENTENENITRILE;1-CYANO-2-BUTENE;pent-3-enenitrile;3-penetenenitrlle;2-Butenyl cyanide;3-Pentenenitrile>2-BUTEN-1-YL CYANIDE
- CBNumber:
- CB0483061
- Molecular Formula:
- C5H7N
- Molecular Weight:
- 81.12
- MDL Number:
- MFCD00001963
- MOL File:
- 4635-87-4.mol
- MSDS File:
- SDS
| Boiling point | 144-147 °C(lit.) |
|---|---|
| Density | 0.842 g/mL at 20 °C(lit.) |
| vapor pressure | 24.9 mm Hg ( 50 °C) |
| refractive index |
n |
| Flash point | 104 °F |
| CAS DataBase Reference | 4635-87-4 |
| FDA UNII | I2GHQ8L4DH |
| EPA Substance Registry System | 3-Pentenenitrile (4635-87-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS02,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H330-H301-H226-H361 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P201-P202-P281-P308+P313-P405-P501 |
| Hazard Codes | T |
| Risk Statements | 10-23/24/25-36/37/38 |
| Safety Statements | 45-41-36/37/39-14 |
| RIDADR | UN 3275 6.1/PG 2 |
| WGK Germany | 3 |
| HazardClass | 3/6.1 |
| PackingGroup | II |
3-PENTENENITRILE price More Price(1)
3-PENTENENITRILE Chemical Properties,Uses,Production
General Description
A clear colorless to amber liquid. Less dense than water. Flash point 104°F. Boiling point 296°F. Generally stable and not liable to polymerization. May be toxic by skin absorption, inhalation or ingestion. Prolonged exposure may result in delayed cyanide poisoning. Avoid direct contact with vapors, mists or liquid. May produce cyanide gas and carbon monoxide during combustion. Used in the manufacture of various organic chemicals.
Air & Water Reactions
Highly flammable.
Reactivity Profile
Nitriles, such as 3-PENTENENITRILE, may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
3-PENTENENITRILE is combustible.
3-PENTENENITRILE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52861 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8672 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50003 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 | market03@meryer.com | China | 40240 | 62 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| TCI (Shanghai) Development Co., Ltd. | 021-67121386 | Sales-CN@TCIchemicals.com | China | 24539 | 81 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44700 | 61 |
| Syntechem Co.,Ltd | info@syntechem.com | China | 12990 | 57 | |
| Sinopharm Chemical Reagent Co,Ltd. | 86-21-63210123 | sj_scrc@sinopharm.com | China | 9823 | 79 |
4635-87-4(3-PENTENENITRILE)Related Search:
1of4