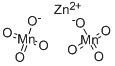ZINC PERMANGANATE
- CAS No.
- 23414-72-4
- Chemical Name:
- ZINC PERMANGANATE
- Synonyms
- zincpermangante;ZINC PERMANGANATE;permanganatodecinc;zinc,dipermanganate;Dipermanganic acid zinc salt;Bispermanganic acid zinc salt;Zinc permanganate hexahydrate.;permanganicacid(hmno4),zincsalt
- CBNumber:
- CB1117255
- Molecular Formula:
- Mn2O8Zn
- Molecular Weight:
- 303.26
- MDL Number:
- MFCD00049618
- MOL File:
- 23414-72-4.mol
| Melting point | 90-105 °C (decomp) |
|---|---|
| Density | 2.450 |
| solubility | soluble in H2O; reacts with ethanol |
| form | black orthorhombic crystals |
| color | black orthorhombic crystals, crystalline; hygroscopic |
| Water Solubility | soluble 3 parts H2O; decomposed by alcohol [MER06] |
| CAS DataBase Reference | 23414-72-4 |
| FDA UNII | 0314CY55NF |
| EPA Substance Registry System | Zinc permanganate (23414-72-4) |
ZINC PERMANGANATE Chemical Properties,Uses,Production
Chemical Properties
Violet-brown or black, hygroscopic crystals. Decomposes on exposure to light and air; soluble in water and acids; decomposes in alcohol.
Uses
Oxidizing agent, medicine (antiseptic).
General Description
A purplish colored crystalline solid. Noncombustible but accelerates burning of combustible material. Explosion hazard if the combustible material is finely divided. Contact with liquid combustible materials may result in spontaneous ignition. Contact with sulfuric acid may result in fires or explosions.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Acetic acid or acetic anhydride can explode with permanganates if not kept cold [Von Schwartz 1918. p. 34]. Explosions can occur when permanganates treated with sulfuric acid come in contact with benzene, carbon disulfide, diethyl ether, ethyl alcohol, petroleum, or organic matter.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Probably a skin, eye, and mucous membrane irritant. Flammable by chemical reaction with reducing agents. A powerful oxidzing agent. When heated to decomposition it emits toxic fumes of ZnO. Used as an antiseptic. See also MANGANESE COMPOUNDS and ZINC COMPOUNDS.
ZINC PERMANGANATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23931 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
View Lastest Price from ZINC PERMANGANATE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-08-12 | ZINC PERMANGANATE
23414-72-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- ZINC PERMANGANATE
23414-72-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd