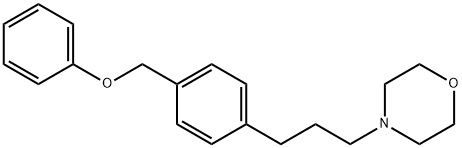
Fomocaine
- CAS No.
- 17692-39-6
- Chemical Name:
- Fomocaine
- Synonyms
- P-652;Panacain;fomocain;Fomocaine;p-Fomocaine;4-(3-(4-(PHENOXYMETHYL)PHENYL)PROPYL)MORPHOLINE;Morpholine, 4-[3-[4-(phenoxymethyl)phenyl]propyl]-
- CBNumber:
- CB11178806
- Molecular Formula:
- C20H25NO2
- Molecular Weight:
- 311.42
- MDL Number:
- MFCD00074897
- MOL File:
- 17692-39-6.mol
| Melting point | 52-53° |
|---|---|
| Boiling point | bp1.1 238-240° |
| Density | 1.0899 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| pka | 7.44±0.10(Predicted) |
| color | Crystals from pet ether |
| FDA UNII | 4XO7A09HQM |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS05,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H318-H302-H400 |
| Precautionary statements | P280-P305+P351+P338-P310-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P273-P391-P501-P264-P270-P301+P312-P330-P501 |
| Toxicity | LD50 i.v. in mice: 175 mg/kg (Nieschulz) |
Fomocaine Chemical Properties,Uses,Production
Originator
Erbocain,Heilit,W. Germany,1967
Definition
ChEBI: Fomocaine is an amine.
Manufacturing Process
64 parts of dry sodium phenolate are dissolved in 300 parts of methylisobutyl
ketone by heating at 110°C. 103 parts of γ-(4-chloromethylphenyl)propyl
chloride are added dropwise with agitation, and the mixture is maintained at
110°C for a period of 4 hours with constant agitation. After cooling, the
reaction mixture is washed 2 or 3 times with 100 parts of water and the
methylisobutyl ketone is distilled off under reduced pressure. The residue is
taken up in 200 parts of petroleum ether and γ-(4-phenoxymethylphenyl)
propyl chloride is crystallized by addition of ice water. The crystals are filtered
off employing a suction pump and dried at 100°C in vacuo (10 mm Hg) for 1
to 2 hours. The γ-(4-phenoxymethylphenyl)propyl chloride melts at 55°C to
56°C after recrystallization from petroleum ether.
130 parts of γ-(4-phenoxymethylphenyl)propyl chloride are heated under
reflux at 140°C for 24 hours with 130 parts of morpholine. The reaction
mixture is treated to give N-[(γ-phenoxymethylphenyl)propyl]-morpholine,
which forms colorless crystals melting at 52°C to 53°C when crystallized from
n-heptane.
Therapeutic Function
Local anesthetic
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx.
Fomocaine Preparation Products And Raw materials
Fomocaine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| Supplier | Advantage |
|---|---|
| Alchem Pharmtech,Inc. | 58 |