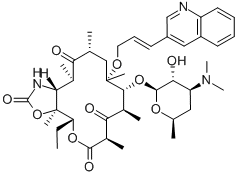
CETHROMYCIN
- CAS No.
- 205110-48-1
- Chemical Name:
- CETHROMYCIN
- Synonyms
- ABT-773;A-195773;CETHROMYCIN;Abbott-195773;quinerythromycin;CETHROMYCIN(ABT-773);11-Amino-11-deoxy-3-oxo-5-o-desosaminyl-6-o-[1'-(3'-quinolyl-2'-propenyl)] erythronolide A 11,12-cyclic carbamate;2H-Oxacyclotetradecino[4,3-d]oxazole-2,6,8,14(1H,7H,9H)-tetrone, 4-ethyloctahydro-3a,7,9,11,13,15-hexamethyl-11-[[3-(3-quinolinyl)-2-propenyl]oxy]-10-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-, (3aS,4R,7R,9R,10R,11R,13R,15R,15aR)-
- CBNumber:
- CB1726437
- Molecular Formula:
- C42H59N3O10
- Molecular Weight:
- 765.93
- MDL Number:
- MFCD08458273
- MOL File:
- 205110-48-1.mol
- MSDS File:
- SDS
| Melting point | 211-213° |
|---|---|
| Boiling point | 927.1±65.0 °C(Predicted) |
| Density | 1.22±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| pka | 10.84±0.70(Predicted) |
| FDA UNII | J0086219X6 |
CETHROMYCIN Chemical Properties,Uses,Production
Description
Cethromycin (formerly ABT-773) is a ketolide antibiotic originally developed by Abbott in the late 1990s, but abandoned in 2002. Since 2004, Advanced Life Sciences has promoted current development of cethromycin. The ketolides are structurally derived from erythromycin A and are designed to overcome resistance to macrolides. One of the main features is the lack of the neutral sugar L-cladinose at position 3 of the erythonolide ring, which is replaced by a keto group (thus giving rise to the class name). Lack of the L-cladinose moiety results in better drug absorption and less gastric irritation through improved acid stability.
Physical properties
Cethromycin is a 6-O-ketolide, meaning that, compared with 11-Nketolides such as telithromycin, linkage with the macrolactone ring is in the 6-O-position, with an O-propylallyl linkage replacing the aminopropyl linkage of telithromycin. Cethromycin presents as a white crystalline powder. As yet, cethromycin has been used only in clinical trials and is not available for clinical use. Development is primarily directed at upper and lower respiratory tract infections.
Uses
Antibacterial.
Definition
ChEBI: Cethromycin is a macrolide antibiotic which displays in vitro activity against both gram-positive and gram-negative bacteria and is currently under investigation for the treatment of community-acquired pneumonia. The US Food and Drug Administration (FDA) have also granted orphan drug designation to cethromycin for the treatment of anthrax prophylaxis, tularemia, and plague (PDB entry: 1NWX). It has a role as an antibacterial drug and a protein synthesis inhibitor. It is a macrolide antibiotic, a member of quinolines and a monosaccharide derivative.
Biological Activity
Oral bioavailability of cethromycin has been poorly studied in humans. Animal studies report values ranging from approximately 36% to 50%. Absorption of cethromycin appears to be dose dependent with time to Cmax increasing from 0.9 to 5.1 hours with increasing dosage. Food does not appear to exert meaningful effects on the pharmacokinetics of cethromycin, but this aspect deserves additional study. In a study on the effects of drug co-administration on the pharmacokinetics of cethromycin, ranitidine was found to significantly reduce cethromycin Cmax concentrations, by 25.7%. Conversely, sucralfate had no effect on cethromycin concentrations. The degree of serum protein binding is considered to approach 90%.
Mechanism of action
The mechanism of action is similar to macrolides and is based on interaction with the peptidyl transferase site of the 50S ribosomal subunit, thus inhibiting the translation of rRNAs and preventing the elongation step of protein synthesis. In addition, cethromycin also interferes at an earlier stage of protein synthesis by disrupting the assembling of 50S subunit precursors to block the formation of a functional 50S subunit. Ketolides in general (including cethromycin) inhibit protein synthesis by binding to the 50S ribosomal subunit, close to the peptidyl transferase site at the entrance of the ribosomal exit tunnel. The presence of a 3-keto group in place of the L-cladinose moiety, common to all ketolides, allows cethromycin to bind to the ribosomal target in the 23S rRNA domain V without causing expression of ribosomal mutations. The additional flexible side chain (in the 6-O position for cethromycin) attached to the macrocyclic ring allows binding to an additional ribosomal site. This dual binding affinity increases the binding affinity of cethromycin several-fold compared with that of erythromycin. It is probably also responsible for overcoming resistance mediated by both the ribosomal mutations (erm) and efflux pump (mef) mechanisms. This leads to an enhanced activity against S. pneumoniae, including most of the macrolide-resistant strains. Conversely, the methoxy group at position C-6 provides greater acid stability than that of other macrolides, and is related to greater gastrointestinal stability.
Drug interactions
Ketolides and macrolides are substrates and inhibitors of the cytochrome P450 (CYP450) 3A4 system. So far, data available specifically for cethromycin are very limited and mainly exist in abstract form. It is expected that interactions are similar to other drugs that are involved in this pathway. This is exemplified by ketoconazole, a potent CYP3A4 inhibitor that caused a 5-fold increase in cethromycin AUC and a 2.5-fold increase in drug Cmax, although cethromycin’s main metabolite showed a decreased Cmax but no effect on its AUC. Similarly, rifampicin (600 mg) significantly affected the pharmacokinetics of cethromycin (300 mg), with a 95% reduction in the ketolide’s AUC and its metabolite N-desmethyl cethromycin.
CETHROMYCIN Preparation Products And Raw materials
Raw materials
Preparation Products
CETHROMYCIN Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | 13119157289@163.com | China | 2971 | 58 |
| Shanghai Boyle Chemical Co., Ltd. | 021-50182298 021-50180596 | sales@boylechem.com | China | 2210 | 55 |
| ShangHai Caerulum Pharma Discovery Co., Ltd. | 18149758185 | sales-cpd@caerulumpharma.com | China | 3427 | 58 |
| Fan De(Beijing) Biotechnology Co., Ltd. | 15911056312 | liming@bio-fount.com | China | 9730 | 58 |
| Nanjing Shizhou Biology Technology Co.,Ltd | 025-85560043 15850508050 | info@synzest.com | China | 9287 | 58 |
| Zhejiang Huida Biotech Co., LTD | 0571-89903882 13626641628 | jiangnan@huidabiotech.com | China | 3656 | 58 |
| Zhejiang Huida Biotech Co., LTD | 0571-0571-89903882 15990081639 | sunshixuan@huidabiotech.com | China | 3705 | 58 |
Related articles
- Cethromycin--- ABT-773
- Cethromycin (formerly ABT-773) is a ketolide antibiotic originally developed by Abbott in the late 1990s, but abandoned in 200....
- Mar 17,2022