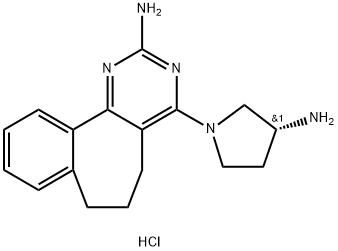
A 943931 2HCl
- CAS No.
- 1227675-50-4
- Chemical Name:
- A 943931 2HCl
- Synonyms
- A-943931 HCL;A 943931 2HCl;A 943931 (hydrochloride)
- CBNumber:
- CB63038491
- Molecular Formula:
- C17H22ClN5
- Molecular Weight:
- 331.85
- MDL Number:
- MFCD16618406
- MOL File:
- 1227675-50-4.mol
A 943931 2HCl price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | A291710 | A943931Dihydrochloride | 1227675-50-4 | 50mg | $695 | 2021-12-16 | Buy |
| ApexBio Technology | B7495 | A943931dihydrochloride | 1227675-50-4 | 50mg | $1410 | 2021-12-16 | Buy |
| ApexBio Technology | B7495 | A943931dihydrochloride | 1227675-50-4 | 10mg | $354 | 2021-12-16 | Buy |
A 943931 2HCl Chemical Properties,Uses,Production
Uses
A 943931 dihydrochloride is a potent and selective H4 receptor antagonist.
Biological Activity
a 943931, is an h4r (one of histamine receptor subtypes) antagonist [1] with high affinities to h4rs of human (ki = 5 nm), rat (ki = 4 nm) and mouse (kb = 6 nm) [2].h4r is one of 4 known g-protein-coupled receptors (h1, h2, h3 and h4 receptors) of histamine for histamine to mediate its physiological functions [3].hmc-1 cells incubated with a 943931 at a concentration of 300 nm for 20 min inhibited the increase in aldh2 activity induced by h4r [4]. in microglia, a 943931 at a concentration of 10 μm partially abolish the release of tnf-α and il-6 induced by histamine at a concentration of 0.1 μg/ml [5]. in bone marrow-derived mast cells, a 943931 inhibited the shape change induced by histamine (ic50 = 0.38 μm) [6].intraperitoneal administration of a 943931 at a dose of 33 μmol/kg potently inhibited itch induced by h4r agonist in mice [6]. in several preclinical models, h4r had been shown to be linked to inflammation [7]. a 943931 had excellent antagonistic activity both in vivo and in vitro across multiple species, displayed good oral bioavailability (90%) and excellent metabolic stability. this compound displays good efficacy in rat pain models and is a good anti-inflammatory agent in mice [8]. a 943931 has an in vivo oral bioavailability of 34% and a half-life of 2.6 h in rats [2]. a 943931 efficaciously reduced acute inflammatory pains induced by formalin in the flinch model and by carrageenan in mechanical and thermal hyperalgesia models in rats [9].
References
[1]. erich h. schneider and roland seifert. the histamine h4-receptor and the central and peripheral nervous system: a critical analysis of the literature. neuropharmacology, 2015, xxx:1-13.
[2]. rogier a. smits, herman d. lim, tiffany van der meer, et al. ligand based design of novel histamine h4 receptor antagonists; fragment optimization and analysis of binding kinetics. bioorg. med. chem. lett., 2012, 22: 461-467.
[3]. huaqing liu, robert j. altenbach, tracy l. carr, et al. cis-4-(piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (a-987306), a new histamine h4r antagonist that blocks pain responses against carrageenan-induced hyperalgesia. j. med. chem., 2008, 51:7094-7098.
[4]. silvia aldi, ken-ichi takano, kengo tomita, et al. histamine h4-receptors inhibit mast cell renin release in ischemia/reperfusion via pkcε-dependent aldehyde dehydrogenase type-2 activation. j. pharmacol. exp. ther., 2014, 349(3):508-17.
[5]. jin zhu, chen qu, xiang lu, et al. activation of microglia by histamine and substance p. cell physiol. biochem., 2014, 34(3):768-80.
[6]. harald engelhardt, rogier a smits, rob leurs, et al. a new generation of anti-histamines: histamine h4 receptor antagonists on their way to the clinic. curr. opin. drug discov. devel., 2009, 12(5):628-43.
[7]. jeffery m cowden, fuqu yu, homayon banie, et al. the histamine h4 receptor mediates inflammation and th17 responses in preclinical models of arthritis. ann. rheum. dis., 2014, 73:600-608.
[8]. rob leurs, paul l chazot, fiona c shenton, et al. molecular and biochemical pharmacology of the histamine h4 receptor. british journal of pharmacology, 2009, 157: 14-23.
[9]. david burns, niu shin, ravi jalluri, et al. annual reports in medicinal chemistry: h4 receptor antagonists and their potential therapeutic applications. burlington: academic press, 2014.
A 943931 2HCl Preparation Products And Raw materials
Raw materials
Preparation Products
A 943931 2HCl Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| AdooQ BioScience, LLC | +1 (866) 930-6790 | info@adooq.com | United States | 2784 | 58 |
| Shanghai Lollane Biological Technology Co.,Ltd. | 021-52996696,15000506266 15000506266 | China | 4182 | 55 | |
| BOC Sciences | -- | info@bocsci.com | USA | 0 | 65 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing1@energy-chemical.com | China | 44843 | 58 |
| MedBioPharmaceutical Technology Inc | 021-69568360 18916172912 | order@med-bio.cn | China | 8141 | 58 |
| Changzhou Furuisi Biotechnology Co., Ltd | 0519-85524369 | 3477467573@qq.com | China | 8618 | 58 |
| Abmole Bioscience Inc. | 021-50967598 02150967598 | sales@abmole.cn | China | 4495 | 58 |
| InvivoChem | 13549236410 | sales@invivochem.cn | China | 6749 | 58 |
| Supplier | Advantage |
|---|---|
| InvivoChem | 58 |
| Aladdin Scientific | 58 |
| AdooQ BioScience, LLC | 58 |
| Shanghai Lollane Biological Technology Co.,Ltd. | 55 |
| BOC Sciences | 65 |
| Energy Chemical | 58 |
| MedBioPharmaceutical Technology Inc | 58 |
| Changzhou Furuisi Biotechnology Co., Ltd | 58 |
| Abmole Bioscience Inc. | 58 |
| InvivoChem | 58 |