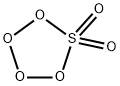
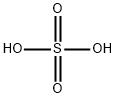
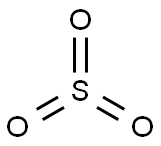
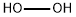peroxomonosulphuric acid
- CAS No.
- 7722-86-3
- Chemical Name:
- peroxomonosulphuric acid
- Synonyms
- Caro acid;Einecs 231-766-6;Sulfomonoperacid;Persulfuric acid;Peroxysulfuric acid;Peroxosulfuric acid;Monoperoxysulfuric Acid;PEROXYMONOSULFURIC ACID;peroxomonosulphuric acid
- CBNumber:
- CB6904889
- Molecular Formula:
- H2O5S
Lewis structure
- Molecular Weight:
- 114.08
- MDL Number:
- MOL File:
- 7722-86-3.mol
- MSDS File:
- SDS
| Melting point | 45, decomposing [HAW93] |
|---|---|
| Density | 2.239±0.06 g/cm3(Predicted) |
| solubility | very soluble in H2O |
| pka | pK2 of Caros acid 9.4 ± 0.1(at 25℃) |
| form | white crystals |
| color | Colorless crystals |
| FDA UNII | UNM59H6037 |
| EPA Substance Registry System | Peroxymonosulfuric acid (7722-86-3) |
SAFETY
Risk and Safety Statements
| Signal word | Danger |
|---|---|
| Hazard statements | H314 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 |
| RIDADR | 1483 |
| HazardClass | 5.1 |
| PackingGroup | II |
peroxomonosulphuric acid Chemical Properties,Uses,Production
Chemical Properties
White crystalline solid; unstable, decomposes at 45°C; commercial product is a syrupy liquid containing equal parts of Caro’s acid and sulfuric acid; stored at dry ice temperature; very soluble in water.
Physical properties
White crystalline solid; unstable, decomposes at 45°C; commercial product is a syrupy liquid containing equal parts of Caro’s acid and sulfuric acid; stored at dry ice temperature; very soluble in water.
Uses
Peroxymonosulfuric acid is used as an oxidizingagent to make glycols, lactones, and esters; formaking dyes; and in bleaching composition.
Uses
peroxomonosulphuric acid (Caro's acid) is used in the preparation of dyes and bleaching agents. It also is used as a strong oxidizing reagent to convert ketones to lactones, to convert olefins to glycols and esters, and to analyse pyridine, aniline and many alkaloids.
Uses
In preparation of dyes; oxidation of olefins to a-glycols; oxidation of ketones to lactones or esters; treating woolens to prevent felting and shrinking; in bleaching compositions.
Preparation
peroxomonosulphuric acid may be prepared by several methods depending on what form of the reagent is desired. Most commonly, it is made by treating potassium perfulfate (K2S2O8) with sulfuric acid. The dry form is prepared by slowly stirring 100 g K2S2O8 into 60 mL of concentrated H2SO4, followed by adding 300 g potassium sulfate. A liquid Caro’s acid is obtained by slowly stirring K2S2O8 into three times the mass of H2SO4. The dilute form of the reagent may be obtained by either mixing K2S2O8 to 40% H2SO4 or by treating K2S2O8 with H2SO4 and adding ice to the mixture.
Alternatively, peroxomonosulphuric acid may be prepared from hydrogen peroxide by treatment with either chlorosulfonic acid or with H2SO4 at –40°C. A 90% H2O2 is used in the preparation.
peroxomonosulphuric acid is a strong oxidizing agent and is very unstable. All laboratory preparations must be carried out in an explosion-proof fume hood under temperature- controlled conditions and in the absence of impurities and oxidizable substances.
Definition
ChEBI: Peroxysulfuric acid is a sulfur oxoacid. It is a conjugate acid of a peroxysulfate(1-).
Hazard
peroxomonosulphuric acid is sensitive to heat and shock. Reactions with organic matter, finely divided metals and other readily oxidizable substances can be violent to explosive. It is a strong irritant to skin, eyes and mucous membranes.
Health Hazard
Peroxymonosulfuric acid is a strong irritant to theskin, eyes, and mucous membranes (Merck 1989).Toxicity data for this compound are not available.
Fire Hazard
Peroxymonosulfuric acid is highly unstable, decomposes dangerously on heating, and evolves oxygen at room temperature. It may react violently with organic matter and readily oxidizable compounds. Violent explosions have been reported with acetone, due to the formation of acetone peroxide (Toennis 1937). It may explode when mixed with many primary and secondary alcohols, manganese dioxide, cotton, many metals in finely divided form, and aromatics such as benzene, phenol, and aniline.
Safety Profile
Strong irritant. Powerful oxidizer. An explosive. Explosive reaction acetone; alcohols; aromatics (e.g., aniline; benzene; phenol); platinum; manganese dioxide; silver. Incompatible with acetone; catalysts; fibers. When heated to decomposition it emits t
peroxomonosulphuric acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Supplier | Advantage |
|---|---|
| Henan Tianfu Chemical Co.,Ltd. | 55 |