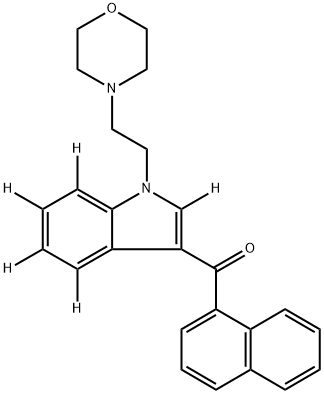
JWH 200-d5
- CAS No.
- 1651833-51-0
- Chemical Name:
- JWH 200-d5
- Synonyms
- JWH 200-d5;JWH 200-d5 (exempt preparation)
- CBNumber:
- CB72591497
- Molecular Formula:
- C25H24N2O2
- Molecular Weight:
- 384.48
- MDL Number:
- MOL File:
- 1651833-51-0.mol
JWH 200-d5 price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 10682 | JWH 200-d5 (exempt preparation) ≥99% deuterated forms (d1-d5) | 1651833-51-0 | 500μg | $79 | 2024-03-01 | Buy |
| Cayman Chemical | 10682 | JWH 200-d5 (exempt preparation) ≥99% deuterated forms (d1-d5) | 1651833-51-0 | 1mg | $150 | 2024-03-01 | Buy |
| Cayman Chemical | 10903 | JWH 200-d5 ≥99% deuterated forms (d1-d5) | 1651833-51-0 | 500μg | $81 | 2024-03-01 | Buy |
| Cayman Chemical | 10903 | JWH 200-d5 ≥99% deuterated forms (d1-d5) | 1651833-51-0 | 1mg | $152 | 2024-03-01 | Buy |
| Cayman Chemical | 10682 | JWH 200-d5 (exempt preparation) ≥99% deuterated forms (d1-d5) | 1651833-51-0 | 5mg | $594 | 2024-03-01 | Buy |
JWH 200-d5 Chemical Properties,Uses,Production
Description
JWH 200-
References
1. Eissenstat, M.A., Bell, M.R., D'Ambra, T.E., et al. Aminoalkylindoles: Structure-
2. Huffman, J.W., Mabon, R., Wu, M.J., et al. 3-
3. Compton, D.R., Gold, L.H., Ward, S.J., et al. Aminoalkylindole analogs: Cannabimimetic activity of a class of compounds structurally distinct from D 9-
4. Pacheco, M., Childers, S.R., Arnold, R., et al. Aminoalkylindoles: Actions on specific G-
5. Bell, M.R., D'Ambra, T.E., Kumar, V., et al. Antinociceptive (aminoalkyl) indoles J. Med. Chem. 34,1099-1110(1991).
JWH 200-d5 Preparation Products And Raw materials
Raw materials
Preparation Products
JWH 200-d5 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Cayman Chemical Company | -- | cayman@caymanchem.com | United States | 6618 | 81 |
| Absin Bioscience Inc. | -- | chenjw@absin.cn | CHINA | 6661 | 58 |
| Shanghai Hao Zhun Biological Technology Co., Ltd. | -- | info@zzsrm.com | CHINA | 6846 | 58 |
| Shanghai Haoran Biotechnology Co., Ltd. | -- | 470003480@QQ.COM | CHINA | 6968 | 58 |
| Resenbio | -- | sales@xarxbio.com | China | 1777 | 50 |
| Beijing Bolide Biotechnology Co., Ltd. | -- | 1985563437@qq.com | CHINA | 6172 | 58 |
| Shenzhen Xinbosheng Biological Technology Co., Ltd. | -- | service@neobioscience.com | CHINA | 6164 | 58 |
| Shanghai Hao Biological Technology Co., Ltd. | -- | market@xlswkj.com | CHINA | 6805 | 58 |
| Beijing Fubo Biotechnology Co., Ltd. | -- | info@f-biology.com | CHINA | 6039 | 58 |
| Shanghai Jijin Chemical Technology Co., Ltd. | -- | jijinhuaxue@sina.com | CHINA | 6617 | 58 |