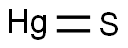Mercury(II) sulfide
- CAS No.
- 1344-48-5
- Chemical Name:
- Mercury(II) sulfide
- Synonyms
- almaden;CINNABAR;vermilion;c.i.77766;Liver ore;vermillion;redcinnabar;pigmentred106;Mercurysulfide;ETHIOPS MINERAL
- CBNumber:
- CB6173856
- Molecular Formula:
- HgS
- Molecular Weight:
- 232.65
- MDL Number:
- MFCD00011046
- MOL File:
- 1344-48-5.mol
- MSDS File:
- SDS
| Melting point | 583.5 °C(lit.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 584°C | ||||||||||||||
| Density | 8.1 g/mL at 25 °C | ||||||||||||||
| solubility | insoluble in H2O; soluble in acid solutions, ethanol | ||||||||||||||
| form | Powder | ||||||||||||||
| color | red | ||||||||||||||
| Water Solubility | Soluble in water (0.00001 g/L). | ||||||||||||||
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Merck | 14,5888 | ||||||||||||||
| Space group | P3121 | ||||||||||||||
| Solubility Product Constant (Ksp) | pKsp: 52.4 | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits |
ACGIH: TWA 0.025 mg/m3 (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
||||||||||||||
| Stability | Stable. | ||||||||||||||
| CAS DataBase Reference | 1344-48-5(CAS DataBase Reference) | ||||||||||||||
| FDA 21 CFR | 310.545 | ||||||||||||||
| FDA UNII | ZI0T668SF1 | ||||||||||||||
| EPA Substance Registry System | Mercury sulfide (HgS) (1344-48-5) | ||||||||||||||
| UNSPSC Code | 12352300 | ||||||||||||||
| NACRES | NA.23 |
| Hardness, Mohs | 3.0 |
|---|
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P362+P364 | |||||||||
| Hazard Codes | N-T,Xi | |||||||||
| Risk Statements | 50/53-36-33-23/24/25-43-31 | |||||||||
| Safety Statements | 61-60-45-36/37/39-28A-26-36/37 | |||||||||
| RIDADR | 2025 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | OX0720000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28521000 | |||||||||
| NFPA 704 |
|
Mercury(II) sulfide price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 243566 | Mercury(II) sulfide red 99% | 1344-48-5 | 50g | $114 | 2024-03-01 | Buy |
| Alfa Aesar | 012992 | Mercury(II) sulfide, black, Puratronic?, 99.999% (metals basis) | 1344-48-5 | 5g | $194 | 2023-06-20 | Buy |
| Alfa Aesar | 012992 | Mercury(II) sulfide, black, Puratronic?, 99.999% (metals basis) | 1344-48-5 | 25g | $708 | 2023-06-20 | Buy |
| Alfa Aesar | 013482 | Mercury(II) sulfide, red | 1344-48-5 | 100g | $207 | 2023-06-20 | Buy |
| Alfa Aesar | 013483 | Mercury(II) sulfide, black | 1344-48-5 | 100g | $163 | 2023-06-20 | Buy |
Mercury(II) sulfide Chemical Properties,Uses,Production
Physical Properties
Mercury(II) sulfide has several modifications, the only two stable allotropic forms are:
1. the red hexagonal form known as cinnabar (alpha form), and 2. the black cubic modification (beta form).
Cinnabar is a red crystalline or powdery substance; hexagonal crystal system; refractive index 2.854; density 8.10 g/cm3; sublimes at 583.5°C; color changes to brown at 250°C and converts to black sulfide at 386°C; reverts to red color on cooling; insoluble in water, alcohol and nitric acid; soluble in aqua regia and solutions of alkali metal sulfides; decomposed by hot concentrated sulfuric acid.
Black sulfide is a black amorphous powder or crystalline substance (beta form); cubic structure; metastable at ordinary temperatures; converts to red sulfide by sublimation at ordinary pressure; density 7.73 g/cm3; melts at 583.5°C; insoluble in water, alcohol and nitric acid; soluble in aqua regia, alkalies, and solutions of alkali metal sulfides.
Preparation
Red sulfide occurs natively and is mined from mineral cinnabar. Also it can be prepared by heating mercury with a solution of potassium pentasulfide, producing a scarlet cinnabar:
Hg + K2S5 → HgS + K2S4
The red sulfide also may be made from black sulfide by heating in a concentrated solution of alkaline polysulfide. The shade of pigment varies with temperature, reaction time, and concentration of the black sulfide.
Alternatively, vermilion may be made by grinding sodium sulfide with sulfur and slowly adding mercury. The shades are not bright when prepared at 0°C.
The black mercury(II) sulfide is prepared usually by precipitation from an aqueous solution of mercury(II) salt with hydrogen sulfide. Thus, when H2S is passed into a solution of HgCl2, a pale yellow precipitate of composition HgCl2•2HgS forms. This converts to amorphous black HgS on further treatment with H2S.
The black sulfide may also be made by other methods such as adding sodium thiosulfate, Na2S2O3 in excess to a dilute solution of sodium mercurichloride, Na2HgCl4 and treating mercury with molten or powdered sulfur.
Reactions
When heated in a current of air, mercury(II) sulfide is converted into metallic mercury and sulfur dioxide:
HgS + O2 → Hg + SO2
Similar reduction to metallic mercury occurs when the sulfide is heated with several metals or metal oxides:
HgS + Fe → Hg + FeS
4HgS + 4CaO → 4Hg + 3CaS + CaSO4
Mercury(II) sulfide dissolves in concentrated solutions of alkali or alkalineearth metal sulfides forming thiosalts, such as Na2[HgS2]•xH2O. Such thiosalts are stable in solution only when alkaline hydroxides are present in excess. These salts also are obtained as bright and deliquescent needles when HgS is heated with sulfur and alkaline hydroxides.
Chemical Properties
Fine, bright-scarlet powder.Insoluble in water and
alcohol.
Cinnabar, HgS, cherry red crystal to 5 mm among snow white rhombic dolomite, CaMg(CO3)2. Wanshan mine, Tongren Pref., Guizhou prov., China.
Uses
Mercuric sulfide (HgS) is a fine, very brilliant scarlet powder that is deadly if ingested. Also known as the mercury ore cinnabar and metacinnabar, it is used as a pigment in the manufacture of paints.
Uses
For coloring plastics, sealing wax, and with FeSO4 for marking linen; manufacture of fancy colored papers; as pigment.
Uses
Mercury(II) sulfide is used in paint, sealing wax and preservatives.
Definition
cinnabar: A bright red mineralform of mercury(II) sulphide, HgS,crystallizing in the hexagonal system;the principal ore of mercury. Itis deposited in veins and impregnationsnear recent volcanic rocks andhot springs. The chief sources includeSpain, Italy, and Yugoslavia.
Air & Water Reactions
Readily oxidized as to be pyrophoric in air [Bretherick 1979. p. 120].
Reactivity Profile
Inorganic reducing agents, such as MERCURY(II) SULFIDE, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive.
Hazard
Highly toxic by ingestion, inhalation, and skin absorption.
Health Hazard
Acute poisoning can result from inhaling dust concentrations of 1.2-8.5 mg/m 3 in air; symptoms include pain and tightness in chest, coughing, and difficulty in breathing. If ingested, toxicity depends on release of t he Hg ++ ion; chronic mercury poisoning can cause kidney, mental, and nervous disturbances. Dust irritates eyes and frequently causes allergic dermatitis; absorption through skin can cause systemic poisoning.
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Yujiang Chemical (Shandong) Co.,Ltd. | +8617736087130 | catherine@yjchem.com.cn | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21626 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29856 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8808 | 58 |
| GLR Innovations | +91 9891111994 | info@glrgroup.in | India | 4535 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8618501500038 | 3899766280@qq.com | China | 26362 | 58 |
| Aladdin Scientific | tp@aladdinsci.com | United States | 57505 | 58 |
Related articles
- Properties and Uses of Cinnabar
- Cinnabar is a toxic mercury sulfide mineral with a chemical composition of HgS. It is the only important ore of mercury. It ha....
- Dec 3,2019
View Lastest Price from Mercury(II) sulfide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-24 | Cinnabar | US $188.00-175.00 / kg | 1000kg | 98% | 20T per month | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-29 | Cinnabar | US $180.00 / kg | 1kg | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-29 | Cinnabar | US $180.00 / kg | 1kg | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. |
1344-48-5(Mercury(II) sulfide)Related Search:
1of4