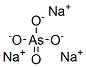비산 나트륨 C화학적 특성, 용도, 생산
생산 방법
For manufacture, ar senic trioxide is mixed with caustic soda. After the addition of a little water, a vigorous reaction begins, spreading quickly through the mixture. Soda may also be mixed with the oxide and a little water, and the resulting mixture dried at elevated temperature in a furnace. In both cases, a water-soluble product is obtained after grinding.
일반 설명
arsenic acid, sodium salt is a colorless to white crystalline solid. arsenic acid, sodium salt is soluble in water. arsenic acid, sodium salt is toxic by ingestion.
공기와 물의 반응
Soluble in water forming weakly acidic solutions.
반응 프로필
Acidic salts, such as arsenic acid, sodium salt , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
건강위험
(Non-Specific -- Arsenic) High mortality rate due to acute poisoning usually within 48 hours.
화재위험
When heated to decomposition, arsenic acid, sodium salt emits toxic fumes of arsenic.
Safety Profile
Confirmed human
carcinogen with experimental tumorigenic data. Poison by ingestion, intravenous, and
intraperitoneal routes. An experimental
teratogen. Other experimental reproductive
effects. Mutation data reported. When
heated to decomposition it emits toxic
fumes of As and Na2O. See also ARSENIC
COMPOUNDS.
비산 나트륨 준비 용품 및 원자재
원자재
준비 용품