무스타르드 기체 C화학적 특성, 용도, 생산
개요
Mustard gas/sulphur mustard is an organic chemical substance synthesised by treating
sulphur dichloride with ethylene. Mustard gas is a chemical substance closely
related to chemical warfare class agents. It is a cytotoxic and vesicant chemical substance,
and exposures are known to cause blisters on the exposed skin. Pure mustard
gas/sulphur mustards are colourless, viscous liquids at room temperature. However,
when used in impure form, such as warfare agents, they appear as yellow brown in
colour. As the name indicates, mustard gas has an odour resembling the garlic, horseradish,
or mustard plants.
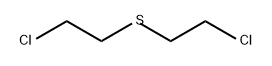
Mustard gas is the common name given to 1,1-thiobis(2-chloroethane), a chemical warfare
agent that is believed to have first been used near Ypres in Flanders on 12 July 1917. Mustard
gas is a thick liquid at ambient temperature. It is heavier than water as a liquid and heavier
than air as a vapour. It does not occur naturally in the environment. Pure liquid mustard
gas is colourless and odourless. It is stable, combustible, and incompatible with strong oxidising
agents. Mustard gas on mixing with other chemical substances appears brown in
colour and gives off a garlic-like smell. When heated, it decomposes and emits highly toxic,
corrosive fumes and fumes of oxides of sulphur and chlorine-containing compounds. It
is soluble in fats and oils, gasoline, kerosene, acetone, carbon tetrachloride, alcohol, tetrachloroethane,
ethylbenzoate, and ether, and solubility in water is negligible. It is miscible
with the OP nerve agents. During earlier years, mustard gas was in use as an important
chemical warfare agent. In fact, it was used in large amounts during World Wars I and II.
Mustard gas was first used by the German army in 1917. It was one of the most lethal of all
the poisonous chemicals used during the war. It was reportedly used in the Iran–Iraq war
in 1980–1988. It is not presently used in the United States, except for research purposes.
화학적 성질
Mustard gas, a chlorinated sulfur compound(s),
is an oily, yellow to black liquid (clear when pure). It has a
sweet, burnt garlic or horseradish-like odor. The odor
threshold for HD is 0.0006 milligram per cubic meter.
용도
Formerly in chemical warfare. In biological studies of alkylating agents.
정의
ChEBI: An ethyl sulfide that is diethyl sulfide in which a hydrogen from each of the terminal methyl groups is replaced by a chlorine. It is a powerful vesicant regulated under the Chemical Weapons Convention.
일반 설명
Mustard gas is a clear amber colored oily liquid with a faint odor of mustard/garlic. Mustard gas is not readily combustible. Its vapors are heavier than air, are very toxic, and can be absorbed through the skin. The effects from exposure to the material include blindness which may be delayed. Prolonged exposure of the container to fire or intense heat may cause Mustard gas to violently rupture and rocket. Mustard gas is also known as dichlorodiethyl sulfide.
공기와 물의 반응
Reacts with water or steam to produce toxic and corrosive fumes(oxides of sulfur and chlorine)
반응 프로필
Mustard gas is incompatible with bleaching powder. Reacts violently with oxidizing materials. Reacts with water or steam to produce toxic and corrosive fumes. Unstable, hydrolyzed in aqueous solution. Avoid high heat; contact with acid or acid fumes. [EPA, 1998].
건강위험
The median lethal dosage is 1500 mg-minute/m3 for inhalation and 10,000 mg-minute/m3 for skin absorption (masked personnel). The median incapacitating dosage is 200 mg-minute/m3 for eye injury and 2000 mg-minute/m3 for skin absorption (masked personnel). Wet skin absorbs more material than dry skin. May cause death or permanent injury after very short exposure to small quantities. It is a blistering gas and is highly irritating to eyes, skin, and lungs. Pulmonary lesions are often fatal. Permanent eye damage and severe respiratory impairment. It is a carcinogen.
화재위험
Can be ignited by large explosive charge. When heated to decomposition, emits highly toxic fumes of oxides of sulfur and chlorine containing compounds. Reacts with water or steam to produce toxic and corrosive fumes. Containers may rupture violently in a fire. Incompatible with bleaching powder. Reacts violently with oxidizing materials. Reacts with water or steam to produce toxic and corrosive fumes. Unstable, hydrolyzed in aqueous solution. Avoid high heat; contact with acid or acid fumes.
Safety Profile
Confirmed human carcinogenwith experimental carcinogenic, neoplastigenic, andtumorigenic data. A human poison by inhalation andsubcutaneous routes. An experimental poison byinhalation, skin contact, subcutaneous, and intravenousroutes. An experimenta
잠재적 노출
Mustard gas is used as an alkylating
agent. It has also been used as a chemical warfare agent,
causing delayed casualties. It is a vesicant and blister agent
in chemical warfare (especially during World War I, military
designation H or HD). Mustard gas is used as a model
compound in biological studies. Mustard gas has been
tested as an antineoplastic agent, but its clinical use as a
tumor inhibitor has been minimal.
Carcinogenicity
Mustard gas is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
운송 방법
UN2810 Toxic liquids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
비 호환성
Sulfur mustard is stable at ambient temperatures.
Reacts with oxidizers (vigorous), strong acids;
acid fumes; strong alkalies; oxygen; water, steam, and other
forms of moisture. On contact with acid or acid fumes, it
emits highly toxic fumes of oxides of sulfur and chlorine.
Rapidly corrosive to brass @ 65℃. Will corrode steel at
a rate of 0.0001 in/month @ 65℃. HD reacts with water;
will hydrolyze; forming HCI and thiodiglycol. When heated
to decomposition (between 149℃ to 177℃), it emits gaseous
hydrogen chloride and oxides of sulfur and chlorine.
Contact with metals may evolve flammable hydrogen gas.
폐기물 처리
Principles and methods for
destruction of chemical weapons: “Destruction of chemical
weapons” means a process by which chemicals are converted
in an essentially irreversible way to a form
unsuitable for production of chemical weapons, and which
in an irreversible manner renders munitions and other
devices unusable as such. Each nation shall determine how
it shall destroy chemical weapons, except that the following
processes may not be used: dumping in any body of water,
land burial or open-pit burning. It shall destroy chemical
weapons only at specifically designated and appropriately
designed and equipped facilities. Each nation shall ensure
that its chemical weapons destruction facilities are constructed
and operated in a manner to ensure the destruction
of the chemical weapons; and that the destruction process
can be verified under the provisions of this Convention
. All decontaminated material should
be collected, contained and chemically decontaminated or
thermally decomposed in an EPA approved incinerator,
which will filter or scrub toxic by-products from effluent
air before discharge to the atmosphere. Any contaminated
protective clothing should be decontaminated using calcium
hypochlorite (HTH) or bleach and analyzed to
assure it is free of detectable contamination (3X) level.
Contaminated clothes and personal belongings should be
placed in a sealed double bag and subsequently placed
inside properly labeled drums and held for shipment back
to the DA issue point. Decontamination of waste or
excess material shall be accomplished in accordance with
the procedures outlined above with the following exceptions:
(a) HD on laboratory glassware may be oxidized
by its vigorous reaction with concentrated nitric acid.
(b) Open pit burning or burying of HD or items containing or contaminated with HD in any quantity is
prohibited. Note: Several states define decontaminated
surety material as a RCRA Hazardous Waste.
무스타르드 기체 준비 용품 및 원자재
원자재
준비 용품