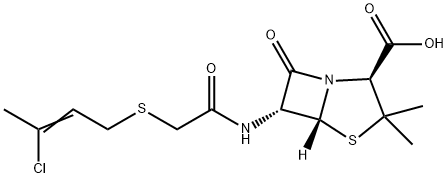
Penicillins C화학적 특성, 용도, 생산
개요
The original fermentation derived penicillins were produced by growth of the fungus
Penicillium chrysogenum on complex solid media, with the result that they were mixtures differing from one
another in the identity of the side-chain moiety. When a sufficient supply of phenylacetic acid is present in
liquid media, this is preferentially incorporated into the molecule to produce mainly benzylpenicillin
(penicillin G in the old nomenclature). Use of phenoxyacetic acid instead leads to phenoxymethyl penicillin
(penicillin V). More than two dozen different penicillins have been made in this way, but these two are the
only ones that remain in clinical use. The bicyclic penicillin nucleus itself is prepared biosynthetically via a
complex process from an acylated cysteinyl valyl peptide.
Veterinary Drugs and Treatments
Natural penicillins remain the drugs of choice for a variety of bacteria,
including group A beta-hemolytic streptococci, many grampositive
anaerobes, spirochetes, gram-negative aerobic cocci, and
some gram-negative aerobic bacilli. Generally, if a bacteria remains
susceptible to a natural penicillin, either penicillin G or V is preferred
for treating that infection as long as adequate penetration
of the drug to the site of the infection occurs and the patient is not
hypersensitive to penicillins.
Penicillins 준비 용품 및 원자재
원자재
준비 용품