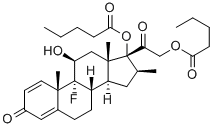
9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate)
|
|
9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate) 속성
- 끓는 점
- 642.3±55.0 °C(Predicted)
- 밀도
- 1.19±0.1 g/cm3(Predicted)
- 산도 계수 (pKa)
- 12.88±0.70(Predicted)
안전
9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate) C화학적 특성, 용도, 생산
Originator
Valisone,Schering,US,1967Manufacturing Process
The valerate is made from betamethasone as a starting material as follows: A suspension of 9α-fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene- 3,20-dione(betamethasone) (2 grams) in sodium dried benzene (500 ml) was distilled vigorously for a few minutes, toluene-p-sulfonic acid monohydrate (30 mg) and methyl orthovalerate (5 ml) were added and distillation was continued for 10 minutes. The mixture was then boiled under reflux for 1.5 hours after which time unreacted betamethasone alcohol (400 mg) was removed by filtration. The benzene solution was treated with solid sodium .bicarbonate and a few drops of pyridine, filtered and evaporated to dryness at about 50°C. The residue, in ether, was filtered through grade III basic alumina (20grams) to remove traces of unreacted betamethasone alcohol, the ether removed in vacuo and the residue of crude betamethasone 17,21- methyl orthovalerate was treated with acetic acid (20ml) and a few drops of water and left overnight at room temperature.The acetic acid solution was poured into water (100 ml) and extracted with chloroform. The chloroform extracts were washed in turn with water, saturated sodium bicarbonate solution and water, dried and evaporated in vacuo. The residual gum was triturated with ether and a white crystalline solid (1.16 grams) isolated by filtration. Recrystallization from ether (containing a small amount of acetone)-petroleum ether gave 9α-fluoro-11β,21-dihydroxy-16β- methyl-17α-valeryloxypregna-1,4-diene-3,20-dione (871 mg) as fine needles.
Therapeutic Function
Corticosteroid9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate) 준비 용품 및 원자재
원자재
준비 용품
9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate) 공급 업체
글로벌( 3)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ZHIWE CHEMTECH CO LTD | 021-20221225 13917446399 |
sales@zhiwe.net | China | 587 | 61 |
9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate) 관련 검색:
베타메타손
BETAMETHASONE BUTYRATE PROPIONATE
[(8S,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-17-(2-methoxyacetyl)-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate
Betamethasone 17-valerate
9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(valerate)
1,3-DIACETOXYACETONE
Betamethasone 21-acetate