Dicumyl peroxide suppliers
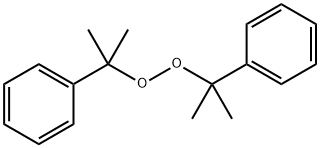
Dicumyl peroxide
- CAS:
- 80-43-3
- MF:
- C18H22O2
- MW:
- 270.37
Company Type
Properties
- Melting point:
- 39-41 °C (lit.)
- Boiling point:
- 130°C
- Density
- 1.56 g/mL at 25 °C (lit.)
- vapor density
- 9.3 (vs air)
- vapor pressure
- 15.4 mm Hg ( 38 °C)
- refractive index
- 1.5360
- Flash point:
- >230 °F
- storage temp.
- 2-8°C
- solubility
- Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly)
- form
- flakes
- color
- White
- Water Solubility
- insoluble
- Hydrolytic Sensitivity
- 4: no reaction with water under neutral conditions
- BRN
- 2056090
- Stability:
- Reacts violently with reducing agents, heavy metals, concentrated acids, concentrated bases. May ignite organic materials on contact. May decompose violently upon exposure to sunlight or if heated. Incompatible with strong oxidizing agents.
- LogP
- 5.6 at 25℃
Safety Information
- Symbol(GHS)




GHS02,GHS07,GHS08,GHS09
- Signal word
- Danger
- Hazard statements
- H242-H315-H319-H360D-H411
- Precautionary statements
- P210-P235-P273-P280-P308+P313-P370+P378
- Hazard Codes
- O,Xi,N,Xn
- Risk Statements
- 7-36/38-51/53-36/37/38-20
- Safety Statements
- 14-3/7-36/37/39-61-14A-45-26-36-17
- RIDADR
- UN 3110 5.2
- WGK Germany
- 2
- RTECS
- SD8150000
- F
- 4.2
- TSCA
- Yes
- HazardClass
- 5.2
- PackingGroup
- II
- HS Code
- 29096000
- Toxicity
- LD50 orally in Rabbit: 4100 mg/kg
Use
The explosive instability of the lower dialkyl peroxides (e.g., dimethyl peroxide) and 1,1-bis-peroxides decreases rapidly with increasing chain length and degree of branching, the di-tert-alkyl derivatives being amongst the most stable class of peroxides. Though many 1,1-bis-peroxides have been reported, few have been purified because of the higher explosion hazards compared with the monofunctional peroxides. Dicumyl peroxide is unlikely that this derivative would be particularly unstable compared to other peroxides in it's class, Bretherick 2nd ed., p 44 1979.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制爆、炸药、化学武器等". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660