Fenpropathrin suppliers
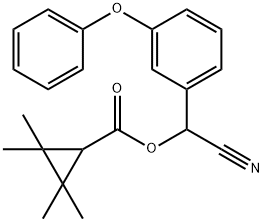
Fenpropathrin
- CAS:
- 39515-41-8
- MF:
- C22H23NO3
- MW:
- 349.42
Company Type
Properties
- Melting point:
- 47 °C
- Boiling point:
- 483.6°C (rough estimate)
- Density
- 1.1700 (rough estimate)
- refractive index
- nD26 1.5283
- Flash point:
- 100 °C
- storage temp.
- -20°C
- solubility
- DMF: 30 mg/ml,DMSO: 30 mg/ml,Ethanol: 15 mg/ml
- form
- A solid
- Water Solubility
- 14.24ug/L(temperature not stated)
Safety Information
- Symbol(GHS)


GHS06,GHS09
- Signal word
- Danger
- Hazard statements
- H301-H312-H330-H410
- Precautionary statements
- P260-P264-P273-P280-P302+P352+P312-P304+P340+P310
- Hazard Codes
- T+,N
- Risk Statements
- 21-25-26-50/53
- Safety Statements
- 28-36/37-38-45-60-61
- RIDADR
- UN 2811 6.1/PG 2
- RTECS
- GZ2090000
- HS Code
- 29269090
- Toxicity
- LC50 (24 hr) in rainbow trout: 76.7 ppb (Coats); LD50 in rats (mg/kg): 2.5 i.v. (Verschoyle); in male, female rats (mg/kg): 24-36, 18-24 orally (Crawford)
Use
A pyrethroid. Fenpropathrin is an ester and nitrile. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "剧毒、有毒、易致癌化学品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660