Pentobarbital suppliers
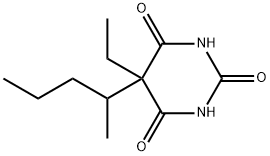
Pentobarbital
- CAS:
- 76-74-4
- MF:
- C11H18N2O3
- MW:
- 226.27
Company Type
Properties
- Melting point:
- 129.5°C
- Boiling point:
- 367.89°C (rough estimate)
- Density
- 1.1376 (rough estimate)
- refractive index
- 1.4620 (estimate)
- Flash point:
- 9℃
- storage temp.
- 2-8°C
- solubility
- Very slightly soluble in water, freely soluble in ethanol. It forms water-soluble compounds with alkali hydroxides and carbonates and with ammonia.
- form
- A neat solid
- pka
- pK1:8.11(0) (25°C)
- Water Solubility
- 679 mg/L
Safety Information
- Symbol(GHS)


GHS06,GHS08
- Signal word
- Danger
- Hazard statements
- H301-H336-H361d
- Precautionary statements
- P201-P202-P261-P264-P270-P301+P310
- Hazard Codes
- T,F
- Risk Statements
- 25-63-39/23/24/25-36/38-23/24/25-11
- Safety Statements
- 7-16-36/37-45-36/37/39-22
- RIDADR
- UN 2811 6.1/PG 3
- WGK Germany
- 3
- RTECS
- CQ5775000
- HazardClass
- 6.1(b)
- PackingGroup
- III
- HS Code
- 2933530000
- Toxicity
- A barbiturate that causes CNS depression, apparently due to a facilitation of GABA-ergic inhibition. It appears that the site of action of pentobarbital may be the macromolecular complex made up of a GABA receptor, chloride channel, benzodiazepine-binding site, and picrotoxin-binding site. Barbiturates have been shown to compete for dihydropicrotoxinin-binding sites. In clinical use, barbiturates such as pentobarbital have been largely replaced as sedative-hypnotics by the much safer benzodiazepines. The sedative-hypnotic properties of barbiturates may lead to abuse, as tolerance and dependence are known to occur. In animals, pentobarbital is routinely used for its anesthetic and anticonvulsant properties, as well as for euthanasia. Pentobarbital, like other barbiturates, can induce the metabolism of other compounds by altering cytochrome P450 activity. The oral LD50 in rats is 118 mg/kg.
Use
Nembutal(Ovation)[Name previously used: Pentobarbitone.];Aethaminalum;Barbityral;Barbopent;Burtylonel;Cafergot p.b.;Calpental;Di-barbs;Dipental;Distonocalm;Ergobel plus;Hypnol;Hypnotal;Hyptonal;Insom rapido;Isoamytal;Isom rapido;Iturate;Narcoren;Nembudeine;Nicaphlogyl;Novarectal;Nova-rectal;Novopentobarb;Novo-pentobarb;Or-trin;Palpent;Pembul;Penbon;Pentalgin;Pentanca;Pentodormol;Pentogen;Pentolos;Petab;Petonel;Praecicalm;Prodormol;Somnophyt;Somnotol;S-spac;Stopp-15;Wans;Wigraine-pb;Yastyl.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "精神麻醉类药品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660