简介
2-溴-4-氯苯甲酸的CAS号是936-08-3,分子式是C7H4BrClO2,分子量是235.46。熔点是157-161°C,沸点是326.5±27.0°C(Predicted),密度是1.809±0.06g/cm3,以及酸度系数(pKa)是2.62±0.10(Predicted)。2-溴-4-氯苯甲酸用于医药、染料载体、增塑剂、香料和食品防腐剂等的生产,也用于醇酸树脂涂料的性能改进[1]。
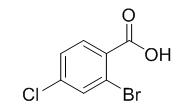
图1 2-溴-4-氯苯甲酸的结构式。
合成

图2 2-溴-4-氯苯甲酸的合成路线[2]。
将硝酸钠(2.21 g)存于水(15 mL)中的水溶液逐滴添加到2-氨基-4-氯苯甲酸(5.00 g,29.1 mmol)和48%氢溴酸(150 mL)存于水中的经搅拌的冰冷却混合物中。将所得混合物在0°C下搅拌2小时。然后用溴化铜(7.81 g)水溶液(20 mL)逐滴处理。添加完成后,将反应混合物加热至环境温度,搅拌过夜。在用乙酸乙酯/己烷(3:1;2 x 400 mL)萃取后,用盐水(200 mL)洗涤结合的有机层,干燥、浓缩,并在二氧化硅(1%甲醇和0.5%氯仿中的乙酸)上进行色谱分析,得到标题化合物2-溴-4-氯苯甲酸。产量4.04克,59%。熔点154-155°C;ES(-)MS m/z 233,(m-H)-与1 Br和1Cl一致。
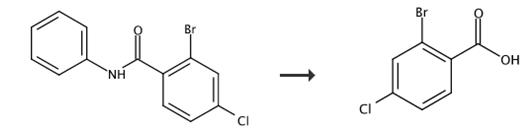
图3 2-溴-4-氯苯甲酸的合成路线[3]。
将粗酸(11a或12a;1.0 g)溶解于浓H2SO4(10 cm3)中,搅拌5 h,并倒入50 cm3冰水中。分离产物并用5%的Na2CO3溶液洗涤。将粗制蒽酮溶解在30 cm3乙酸中。添加2.00 g K2Cr2O7饱和水溶液,并将混合物加热至90°C 1小时。冷却后,将混合物倒入200 cm3水中。过滤分离的固体,在硅胶柱上进行色谱分析(苯,然后是氯仿),然后通过结晶进行纯化得到2-溴-4-氯苯甲酸。13,收率(85.2%)。M、 p.156-158°C,公式:(C7H4BrClO2),分析结果(%):C 35.51,H 1.80,核磁碳谱数据:C(35.71),H(1.71)。红外数据:IR(cm-1):3100(OH)1705(C=0);1H核磁共振δH(200 MHz,二甲基亚砜-d6)=13.5(br s,1H,COOH),7.88(d,1H,J=2.1 Hz,3-A r-H),7.78(d、1H,J=8.4 Hz,5-Ar-H),7.57(dd,1 H,J1=2.1 Hz,J2=8.3 Hz,6-Ar-H”)ppm。
应用
2-溴-4-氯苯甲酸用于医药、染料载体、增塑剂、香料和食品防腐剂等的生产,也用于醇酸树脂涂料的性能改进[4-5]。还可以用于抗真菌及消毒防腐,用作化学试剂及防腐剂。2-溴-4-氯苯甲酸通常用作定香剂或防腐剂。也用作果汁饮料的保香剂。可作为膏香用入薰香香精[6-7]。还可用于巧克力、柠檬、橘子、子浆果、坚果、蜜饯型等食用香精中。烟用香精中亦常用之。以及防腐剂和抗微生物剂[8]。
在工业上,2-溴-4-氯苯甲酸与碱反应可生成5-溴-2-硝基苯甲酸盐,比如2-溴-4-氯苯甲酸与氢氧化钠反应生成5-溴-2-硝基苯甲酸钠。2-溴-4-氯苯甲酸是最常见的一种防腐剂,可用于食品工业以及其他工业[9-11]。2-溴-4-氯苯甲酸与其他碱,比如氢氧化钾,生成5-溴-2-硝基苯甲酸钾。5-溴-2-硝基苯甲酸钠和5-溴-2-硝基苯甲酸钾等苯甲酸的碱金属盐为水溶性。2-溴-4-氯苯甲酸与碱土金属或过渡元素的金属碱性化合物反应生成的盐,比如5-溴-2-硝基苯甲酸钙、5-溴-2-硝基苯甲酸锌、5-溴-2-硝基苯甲酸铜等则为油溶性[12-14]。
泄露处理
2-溴-4-氯苯甲酸一旦被泄露,隔离泄漏污染区,周围设警告标志,切断火源。应急处理人员戴好防毒面具,穿一般消防防护服。用清洁的铲子收集2-溴-4-氯苯甲酸于干燥洁净有盖的容器中,运至废处理场所。如2-溴-4-氯苯甲酸被大量泄漏,收集回收或无害处理后废弃。
参考文献
[1] E.Y. Canales, W.K. Chang, L.P. Debien, P. Jansa, J.A. Loyer-Drew, L.P. Martinez, S. Perreault, G.B. Phillips, H.-J. Pyun, R.D. Saito, M.S. Sangi, A.J. Schrier, M.E. Shatskikh, J.G. Taylor, J.A. Treiberg, J.J. Van Veldhuizen, Preparation of macrocyclic bis-2,2'-azaindole derivatives as inhibitors of peptidylarginine deiminases, Gilead Sciences, Inc., USA . 2021, p. 1052pp.
[2] M. Cybularczyk-Cecotka, J. Predygier, S. Crespi, J. Szczepanik, M. Giedyk, Chemodivergence made possible by micellar photocatalysis: C-H arylation vs. N-dealkylation of chlorinated benzamides in aqueous media, ChemRxiv (2021) 1-16.
[3] J. Farand, J.A. Kaplan, G. Notte, C.L. Olen, M. Sangi, D. Sperandio, Sulfinylaminobenzamide and sulfonylaminobenzamide derivatives as mitochondrial oxidative phosphorylation uncouplers and their preparation, Gilead Sciences, Inc., USA . 2019, p. 254pp.
[4] R. Fleck, J. Proudfoot, Maxi-K potassium channel openers for the treatment of fragile X associated disorders, Kaerus Bioscience Limited, UK . 2021, p. 143pp.; Chemical Indexing Equivalent to 177:67773 (EP).
[5] R. Fleck, J. Proudfoot, Novel maxi-K potassium channel openers for the treatment of fragile X associated disorders, Kaerus Bioscience Limited, UK . 2021, p. 87pp.; Chemical Indexing Equivalent to 177:67774 (WO).
[6] J. Mao, Y. Yang, M. Wang, S. Liu, Y. Wang, D. Chen, B. Han, L. Yu, R. Ma, Method for synthesizing t-butyl 4-chloro-2-bromobenzoate, Changzhou SynTheAll Pharmaceuticals Co., Ltd., Peop. Rep. China . 2019, p. 4pp.
[7] J. Mortier, A. Friberg, V. Badock, D. Moosmayer, J. Schroeder, P. Steigemann, F. Siegel, S. Gradl, M. Bauser, R.C. Hillig, H. Briem, K. Eis, B. Bader, D. Nguyen, C.D. Christ, Computationally Empowered Workflow Identifies Novel Covalent Allosteric Binders for KRASG12C, ChemMedChem 15(10) (2020) 827-832.
[8] D. Nguyen, K. Eis, J.X. Mortier, H. Briem, C. Christ, A.R. Friberg, B. Bader, P. Steigemann, V. Badock, J. Schroeder, F. Siegel, D. Moosmayer, Preparation of [1-(prop-2-enoyl)pyrrolidin-3-yl]-isoquinolin-1(2H)-one, -quinazolin-4(3H)-one, -1,6- or 2,6-naphthyridin-5(6H)-one, -pyrido[3,4-d]pyrimidin-4(3H)-one derivatives as KRAS inhibitors, Bayer Aktiengesellschaft, Germany . 2020, p. 69pp.
[9] S. Shishodia, C.J. Goetz, M.D. Olp, B.C. Smith, Small molecule inhibitors of PBRM1-BD2, and use thereof, The Medical College of Wisconsin, Inc., USA . 2022, p. 145pp.
[10] Y. Wang, H. Li, Preparation method for deuterated macrocyclic compound, Shenzhen TargetRx, Inc., Peop. Rep. China . 2020, p. 57pp.
[11] D.G. Washburn, T.H. Hoang, W.H. Miller, J. Guang, M. Elban, R.S. Davis, M.-H. Ho, J.J. Romano, M. Vimal, M. Ying, Preparation of 2,3-dihydroquinazoline compds. as NAV1.8 inhibitors, GlaxoSmithKline Intellectual Property Development Limited, UK . 2020, p. 551pp.
[12] Q. Yan, X. Shen, G. Zi, G. Hou, Rh-Catalyzed Asymmetric Hydrogenation of α,β- and β,β-Disubstituted Unsaturated Boronate Esters, Chem. - Eur. J. 26(27) (2020) 5961-5964.
[13] X. Yang, H. Lu, X. Zhu, L. Zhou, G. Deng, Y. Yang, Y. Liang, Palladium-Catalyzed Cascade Cyclization of Alkene-Tethered Aryl Halides with o-Bromobenzoic Acids: Access to Diverse Fused Indolo[2,1-a]isoquinolines, Org. Lett. 21(18) (2019) 7284-7288.
[14] Y. Yoshinaga, T. Yamamoto, M. Suginome, Stereoinvertive C-C Bond Formation at the Boron-Bound Stereogenic Centers through Copper-Bipyridine-Catalyzed Intramolecular Coupling of α-Aminobenzylboronic Esters, Angew. Chem., Int. Ed. 59(18) (2020) 7251-7255.
