| Structure |
Chemical Name |
CAS |
MF |
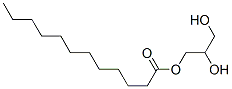 |
Glycerol monolaurate |
40738-26-9 |
C15H30O4 |
 |
beta-Alanine, N-coco alkyl derivs., sodium salts |
68608-68-4 |
unspecified |
 |
Starch Pharm |
|
|
 |
Barm pharm grade |
|
|
 |
Medicinal corn oil |
|
|
 |
MANDRAGORA OFFICINARUM ROOT EXTRACT |
|
|
 |
Sucrose core (medicinal excipients) |
|
|
 |
PolyacrylicResin |
|
|
 |
Pharmaceutical grade Cabo 941 |
|
|
 |
Medicinal potassium acetate |
|
|
 |
High purity medicinal solid sodium methoxide |
|
|
 |
Pharmaceutical grade potassium chloride |
|
|
 |
Medicinal adsorption alumina |
|
|
 |
Poloxamer 407 (pharmaceutical excipient) |
|
|
 |
medical PU foam with perfume |
|
|
 |
humid medicinal materials |
|
|
 |
Cellulose acetate succinate |
|
|
 |
Pharmaceutical grade maltodextrin |
|
|
 |
Medicinal calcium lactate |
|
|
 |
Pharmaceutical grade polyethylene glycol |
|
|
 |
salicylic acid inclusion complex |
|
|
 |
Pharmaceutical grade polyethylene glycol 6000 |
|
|
 |
Oseltamivir Diastereomer I |
|
|
 |
CORN STARCH PHARMACEUTICAL GRADE |
|
|
 |
Vitamin E (DL-Α-tocopherol) (medicinal excipients) |
|
|
 |
99.7% glycerol (medicinal excipients) |
|
|
 |
Egg yolk lecithin (medicinal excipients) |
|
|
 |
Zinc oxide (medicinal excipients) |
|
|
 |
Cross-linked povidone (medicinal excipients) |
|
|
 |
Wheat starch (medicinal excipients) |
|
|
 |
Urea (medicinal excipients) |
|
|
 |
Tannic acid (medicinal excipients) |
|
|
 |
Ammonium sulfate (medicinal excipients) |
|
|
 |
Medicinal hypromellose hollow capsule |
|
|
 |
Mannitol (medicinal excipients) |
|
|
 |
Ethanol (medicinal 95%) (medicinal excipients) |
|
|
 |
Azelaic acid (medicinal excipients) |
|
|
 |
Pharmaceutical film coated premixed adjunct (enteric soluble) (medicinal excipients) |
|
|
 |
Polyacrylic resin latex (gastric collapse) (medicinal excipients) |
|
|
 |
Tetrafluoroethane (internal use) (medicinal excipients) |
|
|
 |
Light magnesium oxide (medicinal excipients) |
|
|
 |
Medicinal hydroxypropyl starch hollow capsule |
|
|
 |
Absolute ethanol (medicinal excipients) |
|
|
 |
Calcium diphosphate (medicinal excipients) |
|
|
 |
Methylparaben (medicinal excipients) |
|
|
 |
L-malic acid (medicinal excipients) |
|
|
 |
Polycarbophil (medicinal excipients) |
|
|
 |
Gluconolactone (medicinal excipients) |
|
|
 |
Red iron oxide (medicinal excipients) |
|
|
 |
Stevioside (medicinal excipients) |
|
|
 |
Medicinal ink (yellow) (medicinal excipients) |
|
|
 |
BEESWAX |
8006-40-4 |
|
 |
783 type medicinal activated carbon |
|
|
 |
Pharmaceutical grade Cabo 934 |
|
|
 |
Medicinal Xylitol |
|
|
 |
Total ionic strength regulator PH5.0-5.5 |
|
|
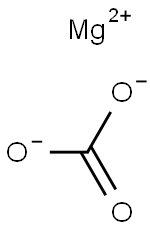 |
magnesium carbonate for medicanaln |
|
CMgO3 |
 |
Pharmaceutical grade polyacrylic resin No. 3 |
|
|
 |
Needle, and a pharmaceutically acceptable activated reagent |
|
|
 |
Pharmaceutical film coating premixes |
|
|
 |
Pharmaceutical sodium sulfite (anhydrous) |
|
|
 |
Gelatin hollow capsule |
|
|
 |
Medicinal dextrin |
|
|
 |
Magnesium stearate (medicinal excipients) |
|
|
 |
Tartaric acid (medicinal excipients) |
|
|
 |
Dimethicone (medicinal excipients) |
|
|
 |
Methanesulfonic acid (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 4000 |
|
|
 |
Pharmaceutical grade povidone K30 |
|
|
 |
Light liquid paraffin (medicinal excipients) |
|
|
 |
Sodium thiosulfate (medicinal excipients) |
|
|
 |
DL-tartaric acid (medicinal excipients) |
|
|
 |
Anhydrous sodium acetate (medicinal excipients) |
|
|
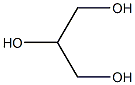 |
Glycerin (for injection) (medicinal excipients) |
|
C3H8O3 |
 |
Magnesium oxide (oral) (medicinal excipients) |
|
|
 |
Ethyl cellulose (medicinal excipients) |
|
|
 |
Ethyl cellulose aqueous dispersion (medicinal excipients) |
|
|
 |
White sugar (medicinal excipients) |
|
|
 |
Polyacrylic resin latex solution for coating premix (medicinal excipients) |
|
|
 |
Pharmaceutical grade povidone K90 |
|
|
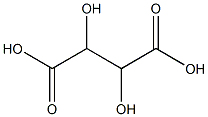 |
Tartaric acid pellet core (medicinal excipient) |
|
C4H6O6 |
 |
Hexadecanol (medicinal excipient) |
|
|
 |
Propylene glycol (medicinal excipients) |
|
|
 |
Medicinal enteric-soluble hypromellose hollow capsule |
|
|
 |
Polyvinyl alcohol (medicinal excipients) |
|
|
 |
Carnitine trisodium (medicinal excipients) |
|
|
 |
Carbomer homopolymer type A (medicinal excipients) |
|
|
 |
Succinic acid (medicinal excipients) |
|
|
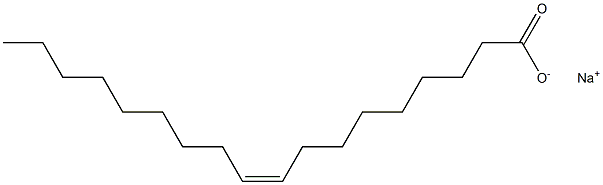 |
Sodium oleate (for injection) (medicinal excipients) |
|
C18H33NaO2 |
 |
Medicinal hollow capsule |
|
|
 |
Activated carbon (for injection) (medicinal excipients) |
|
|
 |
Lanolin (medicinal excipients) |
|
|
 |
Acetic acid-ammonium acetate buffer (pH4.8) |
|
|
 |
Beeswax, synthetic |
71243-51-1 |
|
 |
GOMPHRENA OFFICINALIS ROOT EXTRACT |
|
|
 |
Trihydrate medicinal sodium acetate |
|
|
 |
Medicinal sodium benzoate |
|
|
 |
Soybean oil (for injection) (pharmaceutical excipient) |
|
|
 |
CARBOMER 980 |
139637-85-7 |
|
 |
Medicinal silica gel dryer |
|
|