| Company Name: |
Nanjing Shizhou Biology Technology Co.,Ltd
|
| Tel: |
025-85560043 13675144456 |
| Email: |
sean.lv@synzest.com |
| Products Intro: |
Product Name:Methohexital sodium
CAS:309-36-4
Purity:95%HPLC Package:500mg;1g;5g;10g
|
Related articles - Side effects of Brevital Sodium
- Methohexital sodium is a barbiturate used to make you fall asleep before a surgery or other medical procedure. Brevital Sodium....
- Nov 17,2021
|
| | methohexital sodium Basic information |
| Product Name: | methohexital sodium | | Synonyms: | methohexital sodium;Brevimytal.;SODIUMMETHOHEXITAL;methohexitone sodium;Brevital sodium;Enallynymal sodium;METHOHEXITAL SODIUMQ: What is
METHOHEXITAL SODIUM Q: What is the CAS Number of
METHOHEXITAL SODIUM;Lilly 22451 | | CAS: | 309-36-4 | | MF: | C14H17N2NaO3 | | MW: | 284.289 | | EINECS: | 2062179 | | Product Categories: | | | Mol File: | 309-36-4.mol | 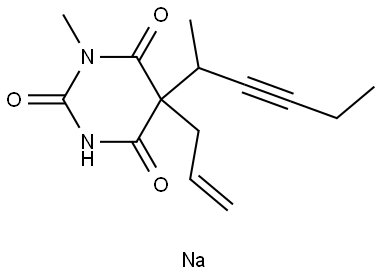 |
| | methohexital sodium Chemical Properties |
| RIDADR | 3249 | | HazardClass | 6.1(b) | | PackingGroup | III |
| | methohexital sodium Usage And Synthesis |
| Description | Methohexital sodium, sodium
5-allyl-1-methyl-5-(1-methyl-2-pentynyl)-
barbiturate, The free acid forms colorless crystals, mp
6 0 – 6 4?C. It is prepared by condensing diethyl
allyl(1-methyl-2-pentynyl)malonate with
N-methylurea . | | Originator | Brevital,Lilly,US,1960 | | Uses | Amobarbital is used as anesthetics for perioperative sedation
as well as in the short-term treatment of insomnia, anxiety,
psychosis, control of seizures, and alcohol withdrawal. These
agents are also exploited in abuse settings. Abuse of barbiturates
started in the 1940s as they were liberally given to military
personnel stationed in the South Pacific in order to help them
cope with harsh conditions. They were called ‘goofballs’ in this
context. After World War II, many servicemen needed detoxification
and treatment for barbiturate dependence upon
returning to the United States. Reported barbiturate abuse
peaked in the 1970s, but has since declined with the increased
use of other sedatives such as benzodiazepines. Use of certain
long-acting barbiturates as anticonvulsants, however,
continues. Phenobarbital is currently the most commonly
prescribed anticonvulsant in use worldwide. Pentobarbital has
been used in the United States for lethal injection during
executions. Phenobarbital is also used for the treatment of
alcohol withdrawal and compares favorably to other agents
including benzodiazepines. | | Uses | Anesthetic (intravenous). | | Definition | ChEBI: The sodium salt of methohexital. | | Manufacturing Process | Preparation of 3-Hexyne-2-ol: A solution of ethyl magnesium bromide was prepared by the reaction of 229 g of ethyl bromide and 48.6 g of magnesium in 750 ml of anhydrous ether. To the ether solution was then added with stirring a solution of 108 g of ethyl acetylene in 250 ml of cold anhydrous ether. The addition required approximately 3 hours, and the mixture was stirred and refluxed for a further period of 3? hours. Thereafter there was added to the reaction mixture a solution of 88 g of freshly distilled acetaldehyde in 170 ml of anhydrous ether, over a period of about 45 minutes and at a temperature in the range of about -10° to 0°C.
The resulting reaction mixture was poured over about 1 kg of crushed ice, and
neutralized with 10% aqueous hydrochloric acid. The organic phase of the
resulting mixture was separated, and the aqueous phase was extracted 3
times with 250 ml portions of ether. The combined organic phase and ether
washings were washed twice with water and dried over anhydrous potassium
carbonate. The dried ether solution was fractionally distilled, and the 3-
hexyne-2-ol formed in the reaction was collected as a fraction boiling at about
79° to 80°C at the pressure of 60 mm of mercury.
Preparation of 2-Bromo-3-Hexyne: A solution of 138 g of 3-hexyne-2-ol and 9
g of pyridine in 138 ml of anhydrous ether was treated with 175 g of
phosphorus tribromide, added dropwise over a period of about 20 minutes at
a temperature of about -10°C. The reaction mixture was permitted to come to
room temperature while stirring for about 3 hours, and was then heated to
refluxing for about 1 hour. After cooling, the reaction mixture was poured over
about 50 g of crushed ice. A two-phase system formed, and the ether layer
was separated, washed with dilute sodium bicarbonate solution, dried over
anhydrous potassium carbonate and fractionally distilled. The 2-bromo-3-
hexyne formed in the reaction was collected at 75°C at the pressure of 50
mm of mercury.
Preparation of Diethyl (1-Methyl-2-Pentynyl) Malonate: To a solution of 28.6 g
of sodium in 430 ml of absolute ethanol were added 200 g of diethyl
malonate. About half of the alcohol was removed by distillation in vacuo, and
thereafter a solution of 200 g of 2bromo-3-hexyne in 100 ml of anhydrous
ether was added slowly to the reaction mixture.
The heat of reaction brought about refluxing during the addition of the 2-
bromo-3-hexyne, and when the addition was complete the reaction mixture
was heated to refluxing for a further period of 30 minutes. A sufficient amount
of water was then added to the reaction mixture to dissolve the sodium
bromide which had formed, and the only organic layer was separated, washed
with water and dried over anhydrous magnesium sulfate. The dried organic
layer was then fractionally distilled under reduced pressure, and the diethyl
(1-methyl-2-pentynyl) malonate formed in the reaction was collected at about
117° to 120°C at the pressure of 2 mm of mercury.
Preparation of Diethyl Allyl (1-Methyl-2-Pentynyl) Malonate: A solution of 12.1
g of sodium in 182 ml of absolute ethanol was prepared, and thereto were
added 126.6 g of diethyl (1-methyl-2-pentynyl) malonate. Most of the ethanol
was then distilled off under reduced pressure, and the residue was cooled and
63.5 g of allyl bromide were slowly added thereto. After completion of the
addition, the mixture was refluxed for about 1 hour. The reaction mixture was
cooled, treated with about 100 ml of water, and the oily organic layer which
formed was removed, washed with water and dried over anhydrous
magnesium sulfate. The dried oily organic material was fractionally distilled in
vacuo, and diethyl allyl (1-methyl-2-pentynyl) malonate boiling at 105° to
107°C at the pressure of 1 mm of mercury was recovered.
Preparation of 1-Methyl-5-Allyl-5-(1-Methyl-2-Pentynyl) Barbituric Acid: A
solution of 23.8 g of sodium in 360 ml of absolute alcohol was prepared and
thereto were added 38.3 g of methyl urea and 96.8 g of diethyl allyl (1-
methyl-2-pentynyl) malonate. The mixture was refluxed for about 20 hours,
cooled, and the ethanol was removed by distillation in vacuo. The residue was
dissolved in about 300 ml of water and the aqueous solution was washed with
ether, and the washings were discarded. The aqueous solution was then
acidified with acetic acid, and extracted with three 150 ml of portions of ether.
The combined ether extracts were washed with 5% aqueous sodium
bicarbonate solution, dried over anhydrous sodium sulfate, and fractionally
distilled in vacuo. The fraction boiling at about 145 to 150°C at the pressure
of 0.5 mm of mercury, weighing 61 g and consisting of 1-methyl-5-allyl-5-(1-
methyl-2-pentynyl) barbituric acid, was collected. The only distillate was
substantially pure, and could be used as such in pharmaceutical preparation
or a salt could be prepared therefrom according to the procedures disclosed
hereinafter. On standing, the oil crystallized. The crystalline 1-methyl-5-allyl5-(1-methyl-2-pentynyl) barbituric acid melted at about 60° to 64°C after
recrystallization from dilute ethanol.
Preparation of Sodium 1-Methyl-5-Allyl-5-(1-Methyl-2-Pentynyl) Barbiturate: A
solution of 61 g of 1-methyl-5-allyl-5-(1-methyl-2-pentynyl) barbituric acid in
100 ml of ether was extracted with 465 ml of 2% aqueous sodium hydroxide
solution. The aqueous extract was washed with successive 75 ml and 50 ml
portions of ether. The pH of the aqueous solution was adjusted to 11.7, using
5% aqueous sodium hydroxide solution. 5 g of decolorizing carbon were
added to the solution with stirring; the mixture was permitted to stand for 20
minutes at room temperature, and the carbon was removed by filtration. A
solution containing 4 g of sodium carbonate in 25 ml of water was added to
the aqueous solution, and the mixture was filtered sterile through a porcelain
filter candle of 02 porosity into sterile bottles. The aqueous solution was then
dried from the frozen state, whereupon a sterile residue of sodium 1-methyl-5-allyl-5-(1-methyl-2-pentynyl) barbiturate, weighing about 62 g was
obtained. | | Brand name | Brevital Sodium (King). | | Therapeutic Function | Anesthetic | | General Description | White powder. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Solutions of methohexital sodium are incompatible with silicone and acid. . | | Fire Hazard | Flash point data are not available for methohexital sodium, but methohexital sodium is probably combustible. | | Clinical Use | Methohexital sodium is a short-acting anesthetic
administered intravenously. It is very potent
and has the shortest duration of action
among the barbiturates used. A disturbing factor
is that rather frequently it causes hiccups directly
after injection. Patients are reported to recover
rapidly from anesthesia. Abuse can lead to addiction. | | Safety Profile | Poison by intravenous
and implant routes. Human systemic effects
by intravenous route: blood pressure
lowering, gastrointestinal effects, and allergc
dermatitis. An FDA proprietary drug.
Cazltion: Excessive use may lead to addction
or habituation. Allergenic effects by
intravenous route. When heated to
decomposition it emits toxic fumes of Na2O
and NOx. See also BARBITURATES. | | Veterinary Drugs and Treatments | Methohexital is sometimes used in small animals as an ultrashort
acting anesthetic agent, but, propofol has largely supplanted methohexital’s
use in small animals. However, because it is not dependent
on redistribution to fat to reverse its effect, it may be useful in
canine sight hound breeds. Because methohexital can induce anesthesia
very rapidly, it may also be useful when general anesthesia
must be administered to a patient with a full stomach, as an ET tube
may be placed rapidly before aspiration of vomitus can occur. |
| | methohexital sodium Preparation Products And Raw materials |
|