| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Norfunalenone
CAS:581786-63-2
Purity:>95% by HPLC Remarks:Norfunalenone is a polyketide fungal metabolite originally isolated from T. stipitatus.
|
| Company Name: |
ChemeGen
|
| Tel: |
18818260767 |
| Email: |
2625930290@qq.com |
| Products Intro: |
Product Name:Norfunalenone
CAS:581786-63-2
Purity:98% Package:10 mg;50 mg;100 mg;500 mg;1 g;5 g;10 g
|
|
| | Norfunalenone Basic information |
| Product Name: | Norfunalenone | | Synonyms: | Norfunalenone;1H-Phenalen-1-one, 2,3,4,7,9-pentahydroxy-6-methyl-;2,3,4,7,9-pentahydroxy-6-methyl-1H-phenalen-1-one | | CAS: | 581786-63-2 | | MF: | C14H10O6 | | MW: | 274.23 | | EINECS: | | | Product Categories: | | | Mol File: | 581786-63-2.mol | 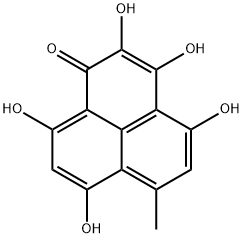 |
| | Norfunalenone Chemical Properties |
| Boiling point | 659.0±55.0 °C(Predicted) | | density | 1.904±0.06 g/cm3(Predicted) | | form | A solid | | pka | 4.50±1.00(Predicted) |
| | Norfunalenone Usage And Synthesis |
| Description | Norfunalenone is a polyketide fungal metabolite that has been found in T. stipitatus. | | Uses | Norfunalenone exhibits weak cytotoxic activity in mouse myeloma NS-1 cell line (ATCC TIB-18) with an IC50 of 70 μM. Norfunalenone also exhibits weak antibacterial activity against B. subtilis (MIC=100 μg/mL; IC50=265 μM)[1]. | | Definition | ChEBI: 2,3,4,7,9-pentahydroxy-6-methyl-1H-phenalen-1-one is a carbotricyclic compound that is funalenone in which the methoxy group at position 2 has been demethylated to the corresponding hydroxy derivative. It is a carbotricyclic compound, a member of phenols and an enone. It is a conjugate acid of a 2,3,4,7,9-pentahydroxy-6-methyl-1H-phenalen-1-one(1-). It derives from a hydride of a phenalene. | | References | [1] Chaudhary NK, et al. Talauxins: Hybrid Phenalenone Dimers from Talaromyces stipitatus. J Nat Prod. 2020;83(4):1051-1060. DOI:10.1021/acs.jnatprod.9b01066 |
| | Norfunalenone Preparation Products And Raw materials |
|