|
6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- Suppliers list
|
| Company Name: |
Haoyuan Chemexpress Co., Ltd.
|
| Tel: |
021-58950125 |
| Email: |
info@chemexpress.com |
| Products Intro: |
Product Name:Nucleoside-Analog-1
CAS:876707-99-2
Purity:>98% Package:2333RMB/2mg
|
6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- manufacturers
- Nucleoside-Analog-1
-
- $198.00 / 2mg
-
2025-10-27
- CAS:876707-99-2
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | 6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- Basic information |
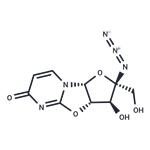
| Product Name: | 6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- | | Synonyms: | 6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)-;Nucleoside-Analog-1;CS-M1995;Nucleoside Analog 1,NucleosideAnalog1 | | CAS: | 876707-99-2 | | MF: | C9H9N5O5 | | MW: | 267.19826 | | EINECS: | | | Product Categories: | | | Mol File: | 876707-99-2.mol | ![6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- Structure](CAS/20150408/GIF/876707-99-2.gif) |
| | 6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- Chemical Properties |
| storage temp. | Store at -20°C | | form | Solid | | color | White to off-white | | Water Solubility | Water : 20 mg/mL (74.85 mM) |
| | 6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- Usage And Synthesis |
| Description | Nucleoside-Analog-1 is a 4′-Azidocytidine analogue against Hepatitis C virus replication. | | Uses | Nucleoside-Analog-1 is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups. | | References | [1]. Smith DB, et al. The design, synthesis, and antiviral activity of 4'-azidocytidine analogues against hepatitis C virus replication: the discovery of 4'-azidoarabinocytidine. J Med Chem. 2009 Jan 8;52(1):219-23. [2]. Smith DB, et al. The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4'-azidocytidine against hepatitis C virus replication: the discovery of 4'-azido-2'-deoxy-2'-fluorocytidine and 4'-azido-2'-dideoxy-2',2'-difluorocytidine. J Med Chem. 2009 May 14;52(9):2971-8. |
| | 6H-Furo[2',3':4,5]oxazolo[3,2-a]pyriMidin-6-one, 2-azido-2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxyMethyl)-, (2R,3S,3aS,9aR)- Preparation Products And Raw materials |
|